
Ins and Outs of Ionics
Keywords
electrolyte,
ion,
random coil.
No, polyelectrolytes are not something you lose when you sweat, nor do you replenish them by drinking a neon-colored sports drink. Those are just plain old electrolytes. But they are related to polyelectrolytes. Here's how: Electrolytes are compounds that like to dissociate or move apart. Put them in water, and they can separate into positive and negative ions. Want an example? Good ol' table salt, NaCl, is an electrolyte. Put it in water and it splits into positive sodium ions and negative chloride ions. That's mainly because water molecules are so good at surrounding and shielding each of the ions so they don't miss their partner counterions so much.
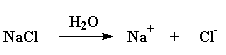
Polyelectrolytes are polymers that do the same thing. They fall apart in water. Want to see one? How about polyacrylic acid? Put this stuff in water and the acid hydrogens split off with the water molecules and form H3O+ ions, like this:
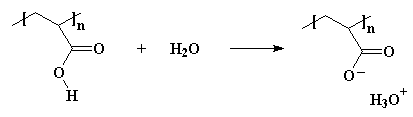
"So what?" you might ask. "Why should I care?"
But when the polymer chain is covered with negative charges (which repel each other), the polymer can't be bunched in on itself. So the chain stretches out, like this:
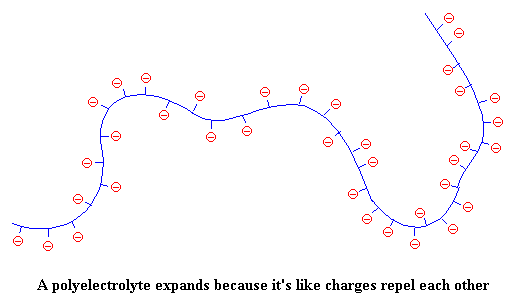
This makes the solution (remember we're talking about polyelectrolytes in solution) much more viscous. Think about it. When the polyelectrolyte chain stretches out it takes up more space, and is more effective at resisting the flow of the solvent molecules around it. So the solution becomes thick and syrupy, and can even gel so that the whole mixture is a semi-solid. Think jello or grape jelly: lots of sugar plus some polymer molecules that make things hard to move around.
But there's a way you can stop this from happening. If you take a solution of a polyelectrolyte in water, and throw in a lot of salt, something fun will happen. The NaCl will separate into Na+ and Cl- ions. In the case of a negatively charged polyelectrolyte like poly(acrylic acid), the positively charged Na+ ions will get in-between the negative charges on the polymer, and cancel them out. When this happens, the polymer chain collapses back into random coil again.
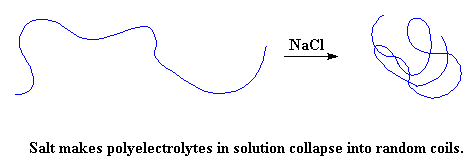
What? You're not sure you believe me? Remember I said this was something you could do, and here's how. Take some hair gel that might be laying around, or you can buy some but get the cheapest you can find. Now put a big glob of it in a bowl. Take a salt shaker, and shake some onto the blob. Let it soak in, dissolve and separate into the ions we talked about at the beginning. When it does this, the gel will collapse quickly into a pretty boring ordinary liquid. This is how dramatic the change in viscosity is when the polymer chains collapse!
What Are These Polyelectrolytes Good For?
Lots of things, in fact. Hair gel, for one. Remember, we were just talking about that. Another important use is in baby diapers. Our good friend poly(acrylic acid) has an unusual property in that it can absorb hundreds of times its own weight in water, or other liquids likely to be found around and in diapers. And that all has to do with the fact that these are "Polyelectrolytes!"
The Best of Both Worlds
Sometimes electrolyte monomers are copolymerized with nonionic monomers. Just a little bit of electrolytic monomer (less than 15%) can change a polymer a lot. We call these copolymers ionomers. Want to know more? Then visit the Ionomer Page.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |