
Fiber Fundamentals
Keywords
crystal,
hydrogen bond,
secondary interaction
So What Are Clothes Made of Anyway?
No, this isn't a page about how oat bran can help reduce the risk of cancer. We're talking about another kind of fiber here. We're talking about polymeric fibers."That's great," you say. "So what's a polymeric fiber?" Since you asked, a polymeric fiber is a polymer whose chains are stretched out straight (or close to straight) and lined up next to each other, all along the same axis, kind of like you see in the picture.

"Ok, so why would I want all my chains lined up like that? Seems pretty artificial." Well, that it is, artificial. At least the way we humans do it. Nature has been doing it like that for billions of years, and doing a bang up job of it, too. Take a look at the graphs below. They show a comparison of the most important physical property of three fibers, their tensile strength.
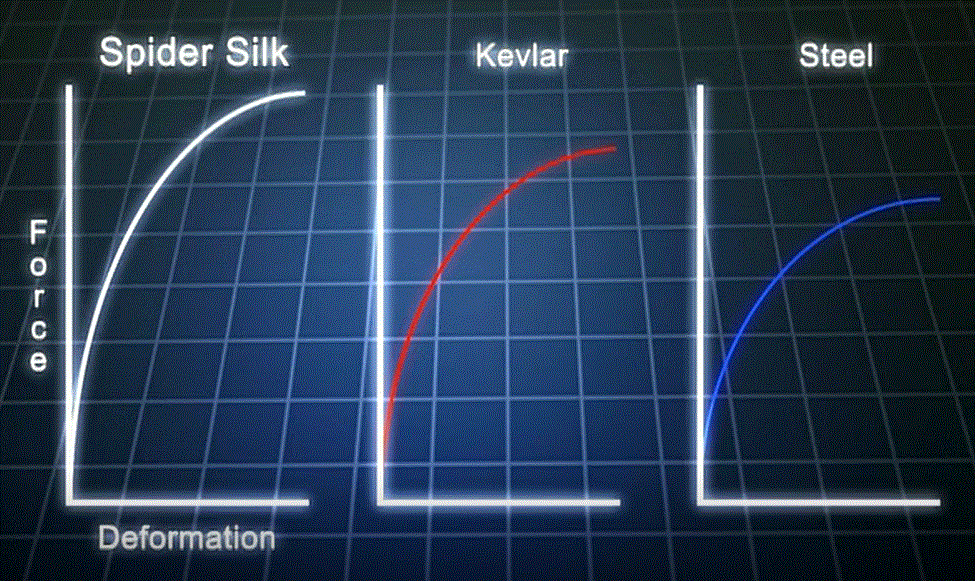
Who would have thought we could make a polymer like Kevlar that's stronger than steel? Go one step further: who would have thought Nature could make a polymer stronger than both steel and Kevlar? No one, for a long time, and this leaves me flabbergasted (neat word from the 1770's). We kind of took spider webs for granted. After all, if you tangle your hand up in one, it's not really all that strong. Ah, but that's based on gross or macroscopic properties. Measure the "specific tensile strength," which takes into account the density and diameter of the fiber, and Bam! You find out how incredible spider silk really is.
Once we figured out how to do the measurements to give us the "true" or "specific" properties, the situation became more complicated. Just how was Nature able to do this? And not only that, Nature made spider silk so that it was also tough. That means it can absorb a lot of energy (in the form of strain) before it reaches its limit of strength. Kevlar and steel are tough also, but for different reasons. Polymers sure get complicated when you start delving into their structures and properties more deeply.
Let's talk more generally about fibers and their properties. Polymers arranged in fibers like these can be spun into threads and used as textiles. The clothes you're wearing are made out of polymeric fibers. So is carpet. So is rope. So is Kevlar bullet resistant vests worn by soldiers and police. Here are some of the polymers which can be drawn into fibers:
- Polyethylene
- Polypropylene
- Nylon
- Polyester
- Kevlar and Nomex
- Polyacrylonitrile
- Cellulose
- Polyurethanes
Quite an array of molecular structures, all the way from the simplest polymer known (polyethylene) to more complicated ones with lots of functionality in and on their chain backbones. It's important to point out that fibers are usually made of polymers which are arranged into crystals. They have to be able to pack into a regular arrangement in order to line up as fibers. In fact, fibers are really a kind of crystal, a really long kind of crystal that has dimensions of length that are hundreds or thousands of times greater than their widths. We can show this by taking a closer look at the way nylon 6,6 packs into a crystalline fiber.

Those hydrogen bonds and other secondary interactions between the individual chains hold the chains together very tightly. So tightly that they don't particularly like to slide past one another. This means that when you pull on the nylon fiber it won't stretch very much, if at all. This is why its fibers are good for using as rope and thread.
But wait! Isn't polyethylene listed there? Yup, and here's a really strange fact: long-chain polyethylene (also called ultra-high molecular weight PE) forms one of the strongest fibers known to man. So strong, in fact, that recent applications include bullet resistant vests and ropes to tie up cruise ships. How can this be without the strong intermolecular forces we just talked about like hydrogen bonding?
Great question. The answer lies in the fact that things can add up. By that I mean that if the chain is long enough and ordered enough through orientation induced crystallization, then even weak van der Waals forces can add up enough to make the PE fibers super-strong. That's why you need perfectly linear polyethylene that's millions of molecular weight in size to achieve high strength PE.
Now back to toughness in hydrogen bonding and nylon-like polymers. Nature used that concept millions of year before man did. I'm talking again about spider silk. The protein molecules that make up spider silk are similar to nylons in that they have the same kind of intermolecular hydrogen bonds. Good and all, but what about the toughness I mentioned earlier? Where does that come from on a molecular scale? The answer lies in how incredibly clever Nature is.
Turns out that, unlike most synthetic polymers used to make fibers that have only one kind of repeat unit, spider silk has several. That makes it a "block copolymer," to put it in synthetic polymer terms. And what's unique about spider silk is that some of those blocks are helical or coil-like; see the figure below. The polymer backbone twists into shapes just like a slinky or a spring. Pull on a spring and it resists some but not like a rod-shaped piece of metal. Let go of the spring, and Bam! it snaps back into its original shape.
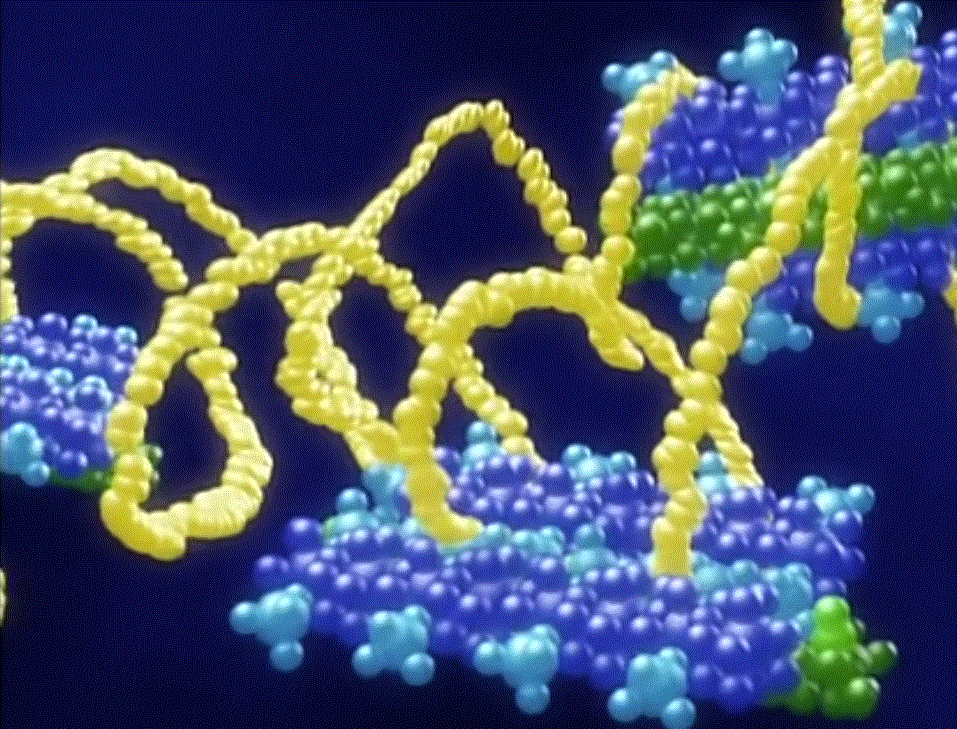
And that, folks, is what makes spider silk such a fantastic fiber. Now that we understand the molecular basis for its properties, we can start engineering synthetic polymers to behave the same way. Then maybe we can have more than a few clingy strands that tangle around our hands and face as we walk through the woods.
A Deeper Look
Ok, let's put things in perspective. The plot below contains dozens of fiber properties. This type of stress-strain curve is one way to determine tensile strength. We can see that most fibers used in fabrics have strengths down in the lower left-hand quadrant. These are useful for most applications like clothes and table cloths. But for high end applications, it's the ones in the upper right-hand quadrant that are most interesting. These include Kevlar and high strength polyethylene (Spectra fiber) that we've talked about. But there are several others we don't have time to go into right now. Keep checking, though, and we'll eventually get to them here at the PSLC.
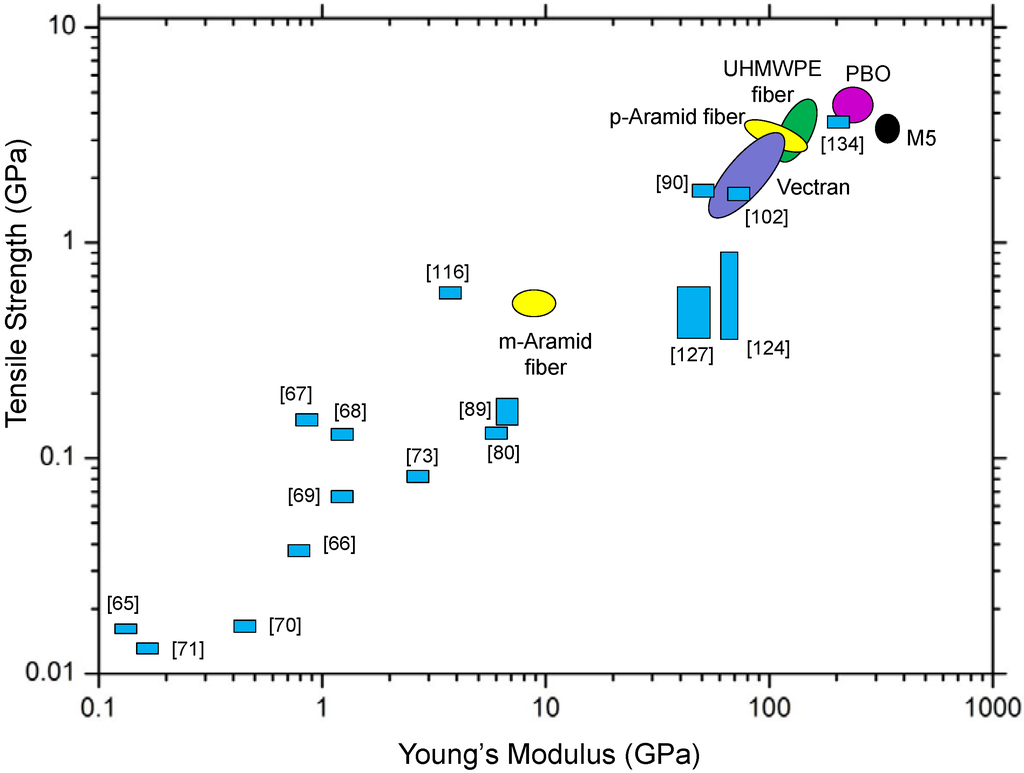
What? You want more now?
In that case I suppose I can tell you that fibers have their drawbacks. While they have good tensile strength, that is, they're strong when you pull or stretch them, they usually have bad compression strength. That is, they're weak when you try to squash or compress them. Also, fibers tend to be strong only in one direction, the direction in which they're oriented. If you pull on them in the direction at right angles to their orientation, they tend to be weak.


|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |