POLYCONDENSATION AND CURING OF EPOXY POLYMERS
Recomended Reading
Objectives
Successful completion of this module should enable the student:
- To gain an understanding of polymerization by the formation of a prepolymer and its curing to form the finished polymer.
- To calculate the epoxide equivalent weight of a prepolymer and the equivalent eight of a curing weight, given structural formulas.
- To differentiate between thermoset and thermoplastic.
Theory
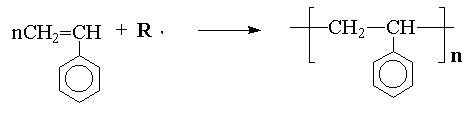
In general, there are two distinctly different types of polymerization, addition and condensation, also called chain-growth and step-growth polymerizations respectively. Chain-growth or addition polymerization may be illustrated by the reaction of styrene with a free-radical:
Condensation or step-growth polymers are formed with the expulsion of a small molecule for each unit added to the polymer chain, and the reaction proceeds in a sequential fashion, forming dimer, trimer, etc. For a polymer such as poly(ethyleneterephthalate) this can be illustrated as follows:

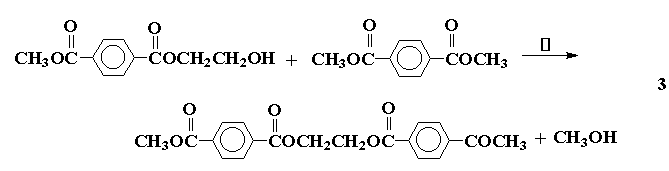
overall:

A type of condensation polymerization that gives a linear polymer (or a "prepolymer" as commonly called) with reactive groups in chain ends, which may be cured to give a network polymer, is the polycondensation of epoxy polymers. The formation of an epoxy polymer may be illustrated by the reaction between 2,2-bis (4-hydroxyphenyl) propane (I) (Bisphenol-A) and epichlorohydrin (II) to initially form a prepolymer as follows:
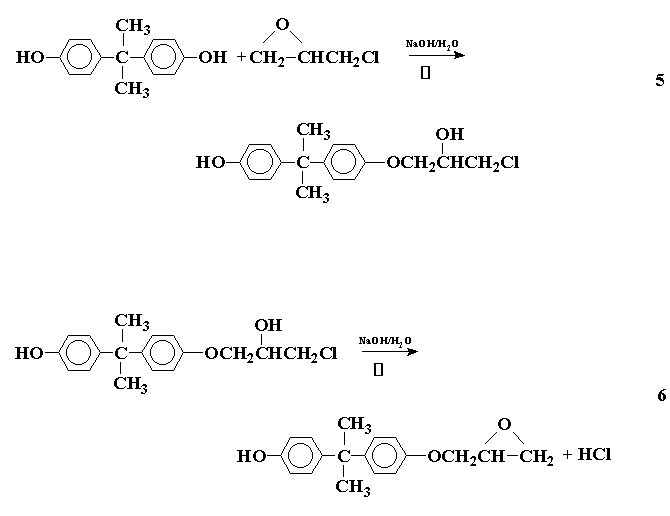
This process may be repeated on either side to give a product that may be represented as:

This is the diglycidyl ether of bisphenol-A (DGEBA), where n represents the number of times the repeating unit occurs in the prepolymer. If n is 0 or 1, the product is a viscous liquid. If n is greater than 1 the product is a brittle solid. The relative amounts of the reactants determine the value of n, a large excess of epichlorohydrin favoring the formation of a liquid.
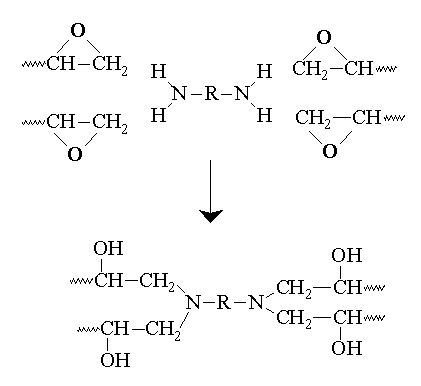
Crosslinking or curing giving a solid is illustrated with a general poly functional amine in Figure 1.
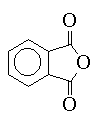
Some anhydrides such as phthalic anhydride (III) are also used for curing agents. They first react with a free hydroxyl group on the chain, freeing a carboxylic acid group to react either with another chain hydroxyl or an epoxide group.
Epoxy resins are thermosetting materials as opposed to thermoplastic materials. Thermosetting plastics are cured or "set" into a form which is retained to the plastic's decomposition temperature without melting while thermoplastics have melting or liquefying temperatures. Thermosetting plastics are also insoluble in their cured stated while thermoplastics usually have several solvents.
The commercial introduction of epoxy resins was in 1947.1 The main use of resins in 1974 was as protective coatings where they are excellent because of the highly unreactive nature of the cured product. Other used include adhesives, laminates, and casting resins. The resins show low shrinkage in their curing reactions which make them extremely valuable in space filling applications.
An epoxy resin system requires two components, a diepoxide or equivalent (epichlorohydrin is equivalent to a diepoxide since another epoxide group is formed upon destruction of the first one) and a reactive diol or polyol. Epichlorohydrin is the most readily available and cheapest diepoxide equivalent, and almost all commercial epoxy systems employ it. Bisphenol-A is also widely used since the aromatic nature enhances the hydroxyl reactivity and adds strength to the resin formed. Aliphatic triol such as glycerol (IV) have been used to give denser crosslinking.
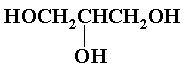
Other polyhydroxylic compounds which find application in epoxy resin formulations are the novolaks, which are products of the reaction of phenols and formaldehyde in acid solution and may be represented by:
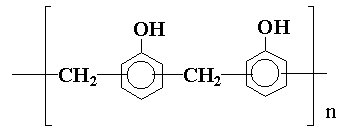
These materials can give epoxy resins of high strength.
The combination of the available diepoxides or equivalents and the polyhydroxy compounds gives a large number of possible epoxy pre-polymers. The number of possible resins is raised even higher by the number of possible curing agents which may be used for each of the prepolymers. Primary amines and anhydrides have been previously mentioned as curing agents, however, other types are available, and the curing may be anionic,
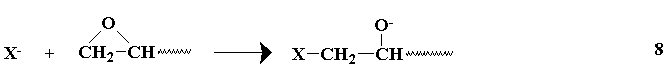
cationic,
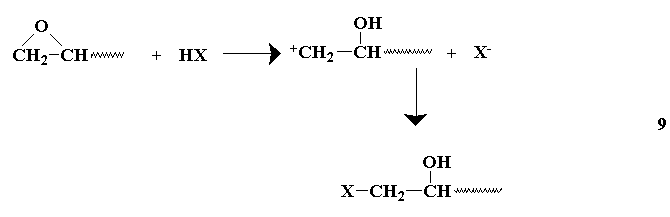
or by Lewis acids or secondary or tertiary amines.

The liberated alkoxide group may react with another epoxide, or abstract the active hydrogen from a reacted epoxy group, providing another anion capable of further reaction with other epoxy groups. The secondary amines undergo reactions similar to tertiary amines after preliminary reactions(similar to primary amines) which form tertiary amines. The choice of a curing agent provides for a wide range of cured resins.
The stoichiometry of the curing reaction should be controlled for optimum properties in the cured resin. The epoxy prepolymers are characterized by the epoxide content or epoxide equivalent weight, which is the weight of resin containing one mole of epoxide groups. For compound (V)
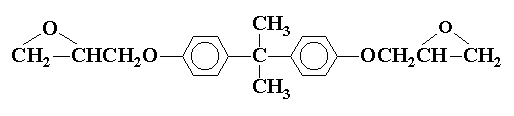
the equivalent weight is the molecular weight divided by two, the number of epoxide groups. The equivalent weight of the curing agent is the molecular weight of the agent divided by the number of sites on the molecule capable of opening epoxy rings. For primary amines this would be the molecular weight divided by the number of replaceable hydrogens. Thus one equivalent of an epoxy prepolymer is cured by one equivalent of a curing agent.
The degree of cure in a resin/curing agent system may be measured by a variety of techniques. Since a large part of the use of epoxies is in areas where their strength is desired, many of the tests such as Vicat softening point and deflection temperature measure the increase in strength of the material.2 Since the material in crosslinked and subsequently not soluble in most solvent systems, the degree of swelling in a solvent may be used to follow the degree of cure.
Experimental
The following reaction to be performed in this experiment may be represented by the following:

This reaction is known as an "advancement" or upgrade" reaction and it is used commercially to convert low molecular weight liquid resin as Epon 829 into higher molecular weight prepolymers which are more suitable for use in coatings and adhesives. The product to be made in this laboratory is useful as an adhesive.
In order to determine the ratio of reactants to be used in the synthesis, it is useful to employ the Carother's equation in the form shown below.
| where: | A=weight of resin to be advanced |
| B=equivalent weight of EPON 829 resin | |
| C=weight of Bisphenol A | |
| D=equivalent weight of Bisphenol A | |
| E=desired equivalent weight of prepolymer |
The equivalent weight of the resin used will be provided by the lab instructor and the equivalent weight of high purity Bisphenol A is taken to be one-half the molecular weight, or 114 g/mole. The student will calculate the percentage of reactants required to produce a weight per epoxide (equivalent weight) of 300 g/eg for the prepolymer.
- Set up a reaction kettle with stirring motor, thermometer, and heating mantle in the mantle in the usual fashion (a condenser is not required.) Whenever gas rod has to be inserted into a rubber stopper the glass should be lubricated with glycerin, both hands should be wrapped in towels so that in the event of the glass breakage the towels would protect the hands, and the glass should be inserted with an easy twisting motion.
- Add the weight of resin calculated previously to obtain a 300 gram total reaction mixture, and begin heating with moderate agitation.
- At temperature of 100oC, add the Bisphenol A to the kettle. Continue heating until all the Bisphenol A is dissolved.
- After 2 hours of heating, pour the reaction mixture into a container specified by the lab instructor. Save the final solution sample used for the WPE determination.
- Clean up the reactor by dissolving the residual resin with acetone. This is most easily accomplished while the flask is still warm, as the resin is difficult to dissolve when cooled to room temperature.
Prepare two Erlenmeyer flasks by pipetting 20 ml of standardized HCl in pyridine (which is prepared by diluting 16 ml of concentrated HCl to one liter with pyridine) into each flask. Accurately weigh approximately 0.75 g of the solution sample saved from step 5 by difference into each flask using the Mettler balance. Weight-by-difference is accomplished by weighing the tube when full, pouring the specified amount into the flask and reweighing the tube. The difference in the two weights in the weight added to the flask.
The flasks are heated gently on a hot plate at a setting of 2 for 20 minutes. Six drops of phenolphthalein indicator are added, and the samples are titrated to a phenolphthalein endpoint with 0.1 N potassium hydroxide in methanol. Care should be taken during titration, as the solutions turn cloudy prior to the color change.
The WPE is calculated from the following formula:
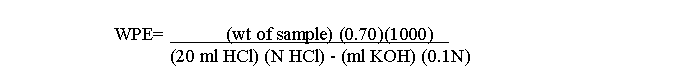
(NOTE: ALL WORK WITH PYRIDINE SHOULD BE DONE IN A HOOD. ALSO, CARE SHOULD BE TAKEN TO AVOID EVAPORATION OF THE METHANOL IN THE POTASSIUM HYDROXIDE STANDARD.)
This product will be tested for adhesion during the next lab period.
Adhesion Sample Preparation
Four adhesion samples will be prepared in the following manner:
| 1. | In a plastic beaker, weigh 50 g of the epoxy resin sample. | ||||||
| 2. | Calculate the desired amount of curing agent (CA) for the samples by using the following formula: | ||||||
| grams resin | x | wt per active | x | ratio | = | grams | |
| WPE resin | hydrogen in CA | multiplier (1) | CA | ||||
| The weight per active hydrogen in CA is determined by calculating the molecular weight and dividing by the number of active hydrogens. The ratio multiplier is the second value in the epoxy:curing agent ratio which will be assigned by the instructor. | |||||||
| 3. | Weigh the CA into the plastic beaker with the epoxy and mix thoroughly. The mixture should be slightly hazy after mixing. | ||||||
| 4. | Coat a substrate supplied by the instructor with the mixture such that the dry film thickness will be about 2 mils thick. | ||||||
| 5. | Remove excess adhesive from the sides of the stud with a wooden applicator. | ||||||
| 6. | Place the coated substrate in an oven, which has been preheated to 100oC, and cure for 1 hour. | ||||||
An Instron model 1140 will be used to measure the strength of the adhesives made in this experiment. A brief discussion of the Instron is included as an appendix to this section. The steps in the test are also included in the appendix. The adhesive will be tested using the following parameters:
| Load Cell: | 500 Kg |
| Chart Speed: | 20-50 cm/min |
| Crosshead Speed: | 5 cm/min |
| Full Scale: | 100 Kg |
The test is complete when the pen returns to zero stress on the chart. Inspect the tested area and determine the type of coating failure, rating it according to the following scale:
| A | adhesive failure of coating at substrate |
| C | cohesive failure in coating |
| AC | combination of A and C |
| S | adhesive failure at stud |
| CS | combination of C and S |
Calculate the tensile strength needed to cause this failure.
Tensile Property Calculations
TENSILE STRENGTH (T.S.)=Force/Area
| T.S.= | (full scale value, kg)(% full scale @ break)(accel. Due to gravity, g) |
| (thickness of sample, cm)(width of sample, cm) |
| example: | T.S.=(200bkg)(a)(9.8 m/sec2)/wT |
| T.S.=(200 kg)(0.51)(9.8 m/sec2)(1.31 cm)(0.323 cm) | |
| T.S.=2362 g.m/s2cm2=2.362x103 N/cm2 | |
| ELONGATION= | 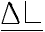 =distance jaws moved (inches) =distance jaws moved (inches) |
| Lo=initial distance of jaws (inches) |
| example: | Elongation= | d(chart speed/crosshead speed) |
| Lo | ||
| Elongation= | 3.5in (5cm/min/10 cm/min) | |
| 4.0 in | ||
| Elongation=0.43 | ||
| Elongation=43% | ||
| MODULUS= | Initial Stress | |
 |
||
| example: | Modulus= | (f.s. value)(% f.s. at break)(g)/w.T |
| [d' (chart speed/crosshead speed)]/Lo | ||
| Modulus= | (200 kg)(.8)(9.8 m/s2)(1.31 cm)(0.323 cm) | |
| {(1.1in)(5cm/min/10cm/min)}/4in | ||
| Modulus= | 26,950.7 kg.m/s2.cm2 | |
| Modulus= | 2.7 x 104 N/cm2 | |
b 200 kg is obtained by having 500 kg lead cell and using a range setting of 20 M.
Figure 3
Instron Chart of Tensile Speciment

Figure 4
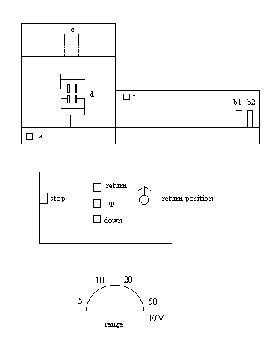
Operational Parts of a Tensile Tester
Calibration and Set-Up of the Instron Tensile Tester
- At least 15 minutes prior to use, turn on both switches marked "A" in figure 4.
- Install the desired load cell in the upper bar marked "E" in position "C". Next, place proper jaws, restraining devices, or upper coupling adaptor n proper "D" position.
- Install proper gears for chart speed and crosshead speed. Be sure all gears are completely stopped before changing them.
- Set the Range to M. This is the metric range and the numbers
represent the following percentages of the load cell:
5-10%
10-20%
20=40%
50=100% - If a sample is to be secured in place between parts "D", do so now.
- Turn on the PEN switch, B-1, figure 4. Swith the RANGE dial to ZERO and zero the pen on the LEFT side (zero line) of the chart paper using the ZERO dial.
- Turn the RANGE dial to 20M and adjust the pen on the same line as in 6 above using the COARSE and FINE BALANCE adjustment dial.
- Press the RED button on the load cell "C", figure 4. Calibrate the pen to the RIGHT side of the paper using the CALIBRATE dial.
- Calibration is now complete. Set the RANGE for the desired percent of the load cell for full scale reading.
- If the sample is ready to be tested, turn the PEN switch ON and the CHART switch to CONTINUE. These switches are B1 and B2, figure 4.
- Push the UP button, figure 4.
- At the desired test result, push the STOP button, figure 4.
Post Test
- Calculate the n value of the epoxy product A in the upgrade reaction if the WPE is 300; 900; 1800. Determine a formula which will allow you to calculate this value (which is also equal to the number of hydroxyl groups). Calculate the combining ratio for a curing reaction between EPON 829 (WPE = 198) and phthalic anhydride.
- Predict the strength and flexibility properties at room temperature and at 150oC for the thermoset polymer resulting from the reaction between EPON 829 and the following curing agents.
