INTRODUCTION:
Two experiments are actually combined into one through the concept of rings or cyclic structures, either in the monomer or the polymer formed. Seems a little tenuous, but these procedures are short and it does give you a different perspective on things. Background information is given on the general concepts of ring-opening and ring-forming polymerizations that includes industrial examples and mechanisms. The polymer synthesis for each type is combined with characterization by IR spectroscopy and dilute solution viscosity. The latter illustrates the polyelectrolyte effect for the charged ammonium polymer in water.
Polyamines, polyamine salts and polyamides are used commercially in a wide range of applications. These include sizing for paper and textiles; recovery and recycle of trace metal contaminates from chemical plants; and flocculation of particulate matter for water clarification. These polymers are strong complexing and chelating agents for metal salts, and such complexes have been used for a variety of catalytic applications. In addition, many of the polyamine compounds are basic catalysts in their own right or in conjunction with organic comonomers, and have been used to make a variety of synthetic chemicals.
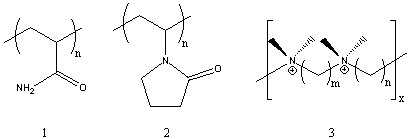
Several of the presently available commercial polymers containing amide and ammonium functionality are given in Figure 1. These included vinyl addition polymers from acrylamide (1) and N-vinylpyrrolidone (2), and step-growth polymers containing quaternary ammonium groups (polyionenes, 3) obtained from polycondensation of diamines and bishalides.
Figure 1. Commercial Amide and Ammonium Polymers.
In this experiment, we examine two major types of polymerization processes involving heterocyclic monomers or repeat units. Polymer synthesis involves ring-opening polymerization to yield a polyamide from an oxazoline, and cyclopolymerization of diallylamine derivative to yield a polymer containing pyrrolidine units.
Ring-opening polymerization mechanisms There are several commercially important polymers which are synthesized via ring-opening polymerization. Examples summarized in Figure 2 include such common polymers as polyoxyethylene (POE, 4), poly(butylene oxide) (PBO, 5), nylon 6 (6), and poly(ethyleneimine) (PEI, 7). This last polymer is obtained by a non-selective process which can involve attack on the ethyleneimine monomer by either chain-ends or internal secondary amines of the growing polymer. These competing reactions lead to a highly branched polymer structure which contains primary, secondary and tertiary amine units.1
Figure 2. Common Ring-Opening Polymerizations.
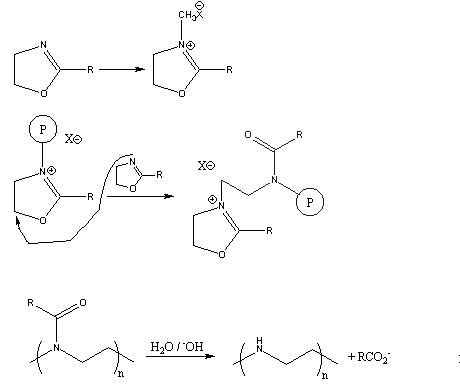
Several years ago, a novel synthesis of completely linear PEI was developed.2 The method utilized a ring-opening polymerization also, but of a 5-membered heterocycle that resulted in formation of a substituted amide rather than the free amine obtained from ethyleneimine. Figure 3 summarizes the initiation and propagation steps for this polymerization 3 as well as the hydrolysis reaction and the final polymer structure.
Figure 3. Ring-Opening Polymerization Mechanism for 2-Substituted Oxazolines
and Subsequent Polymer Hydrolysis; the Circled "P" Represents Polymer
Chain with the Indicated Active Chain End.
This method gives linear PEI (8) by a two-step process. In addition, the intermediate polymers containing amide functionality have become important in their own right.4 Many of these polymers are being examined for various unique applications involving a combination of properties. Many are soluble both in water and in a wide range of organic solvents. The amide functionality provides multiple sites for complexation and chelation of a variety of metal salts. The controlled spacing of the pendent amide derivatives along the 3-atom repeat unit in the backbone provides a novel alternative to the normal 2-atom backbone obtained with vinyl polymerization (see for example poly-(N-vinylpyrrolidone), 2). Finally, partial hydrolysis can give polymers containing both amide and amine or ammonium groups which can interact with substrates, together or in a sequential fashion.
The most common oxazoline derivative available today is the 2-ethyl compound. In this experiment, the monomer is polymerized using a cationic initiator to give high molecular weight polymer which is characterized by both IR and solubility behavior.
Cyclopolymerization
Cyclopolymerizations were first discovered by Professor George Butler in the late '50's.5 Since then, a wide variety of monomers have been found to undergo cyclopolymerization. We concentrate here on a diallylamine derivative. The cyclopolymerization process involves formation of a heterocyclic ring during polymerization as illustrated in Figure 4. The monomer shown, diallyldimethylammonium chloride, is one of the most widely used commercial derivatives.
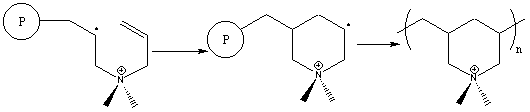
The cyclopolymerization mechanism involves two sequential propagation steps.6 Intermolecular attack of a propagating radical is immediately followed by an intramolecular attack to form the heterocycle. Surprisingly, this second step leads to the unstable primary radical through kinetic rather than thermodynamic control, and is followed by immediate reaction with another monomer molecule. Two possible side reactions can occur in these polymerizations, involving crosslinking and chain transfer, but they are not observed. In general, cyclopolymerization of diallylammonium compounds proceeds cleanly to high molecular weight with no crosslinking.
Figure 4. Free Radical Cyclopolymerization Mechanism of a Diallylammonium Monomer
Figure 5. Free Radical Cyclopolymerization Alternate Mechanism of a Diallylammonium Monomer.
One of the earlier drawbacks in such polymerizations involved the use of peroxide initiators. Extensive yellowing of the product polymer and inefficient initiation lead to low yields and undesirable properties. A recently reported improvement on these polymerizations involves a new commercial initiator V-50 (2,2'-azobis(2-amidinopropane . 2HCl, 9). This water-soluble species cleanly forms carbon radicals that initiate diallylammonium cyclopolymerization to high yield.7
In this experiment, the polymerizability of diallyldimethylammonium chloride is examined. The polymer is purified by precipitation from water and characterized by dilute solution viscosity.
EXPERIMENTAL:
Polymerization of 2-EthyloxazolineA clean, dry test tube is fitted with a rubber septum fastened on with wire. Approximately 2 ml of 2-ethyloxazoline8 is injected into the test tube which is then suspended in an oil bath preheated to 120oC. After a few minutes equilibration, the test tube is carefully removed and approximately 5ml of dimethylsulfate8 is injected. The test tube is put back in the oil bath. The solution gradually becomes more viscous until it gels or solidifies (about 2 h). The test tube is removed from the oil bath and allowed to cool. After removing the septum, 5 ml of methylene chloride is added to dissolve the mixture. This solution is then poured slowly into 50 ml of rapidly stirring mixed hexanes. The solvent is carefully decanted from the solid polymer which is washed again with more hexanes and finally isolated by filtration. CAUTION: dimethylsulfate is toxic and should be handled only in small quantities with good ventilation. Polymerization of Diallyldimethylammonium Chloride
Commercial monomer is usually available at 65 wt-% solution in water.9 This is suitable for direct polymerization. Approximately 5 ml of this solution is added to a test tube along with initiator 9 (V-509, about 0.05 g, ca. 1 mol-%). A septum cap is wired in place and the reaction mixture purged for 5-10 min with N2 through inlet and outlet needles in the septum. The test tube is placed in a preheated water or oil bath at 60-65oC. A small diameter syringe needle is left in the septum to relieve pressure from liberated N2 gas. Polymerization takes place rapidly to give a gelled or solid mass within 1-2 h. The polymer is isolated by precipitation into 100 ml ethanol stirring rapidly in a 250 ml beaker. Purification can be carried out by reprecipitation from water into ethanol.
DISCUSSION:
The two synthetic procedures are straight-forward and can be carried out with a minimum of special preparations and precautions. However, dimethylsulfate is toxic. Only enough material for immediate use should be used. The polymers are important commercially and represent less well-known specialty chemicals. In addition, their synthesis introduces the student to heterocyclic compounds in the context of polymer formation.
Two synthetic extensions of the experiment are possible. One involves synthesis of a poly(diallylamine)7, a polymer that is more difficult to purify and characterize. (Diallylamine is also toxic). Alternatively, the oxazoline polymer can by hydrolyzed in refluxing aqueous acid and neutralized to obtain the free amine polymer. These two polymers can then be compared with the other amide and amine polymers made in this experiment.
Polymer characterization involves qualitative evaluation of solubility behavior, dilute solution viscosity, and IR spectroscopy. Solubility should be evaluated for common organic solvents, acetic acid, and aqueous acid and base solutions. The results can be compared with other available polymers. The students must be made aware of the importance of allowing sufficient time for dissolution and swelling to take place (5-12 h). Unlike low molecular weight materials which normally dissolve rapidly or not at all, polymers take appreciable time to untangle and move away from the solid polymer mass. This effect becomes more pronounced the higher the molecular weight of the polymer.
Dilute solution viscosity is one of the most common and useful initial characterization techniques for polymers. At the very least, a viscosity value of more than ca. 0.1 dL/g tells you that you do have a polymer. More important, qualitative comparisons are possible for polymers of the same composition; ie., increasing viscosity values correlate directly with increasing molecular weight and polymer size. Detailed procedures have been published previously for viscosity determinations.
One very interesting aspect of the viscosity behavior of poly(diallyldimethylammonium chloride) is the polyelectrolyte effect that it shows.12 While well-behaved polymers show a linear relationship with respect to concentration, polyelectrolytes usually show higher reduced viscosity with decreasing concentration. This is demonstrated in the upper plot of Figure 6 (viscosity with units of dL/g plotted against concentration in g/dL). Addition of electrolytes (NaCl) at relatively high concentrations ( > 0.5M) compensates for the polyelectrolyte effect by masking the electrostatic repulsion of cationic groups along the polymer backbone. This is shown in the lower portion of Figure 6 where plots to two different types of viscosity values10,11 for the polymer plus electrolyte show linear behavior.
IR spectroscopy is the most routine spectral characterization technique available for polymers. The formation and IR characterization of polymer thin films is facile,13 giving both qualitative14 and quantitative15 information. Figure 7 gives the spectrum of the oxazoline polymer. Functional group identification can be required of the students, although polymer spectra often display unexpected combination bands and contaminant peaks from retained solvent and reactants.



 Return to
Macrolab Directory
Return to
Macrolab Directory