Organic pigments are intensely colored, particulate organic solids that are essentially insoluble in, and physically and chemically unaffected by, the vehicle or substrate into which they are incorporated.
Color is produced in compounds by selective absorption and reflectance of specific wavelengths of the visible spectrum. Selective effect arises from the presence of vibrating (resonating) electrons in the strucure of a molecule or molecular group. If a molecule absorbs in the visible range, it possesses a color complementary to that which is absorbed. Thus, a compound absorbing in the violet is seen as yellow. Chemical groups which cause absorption and give rise to color are called chromophores. Groups which intensify or modify color are known as auxochromes.Characteristics of Organic PigmentsBright, pure, rich colors
More expensive than inorganic pigments
Less resistant to sunlight, humidity, and chemicals
Key raw materials are petroleum based
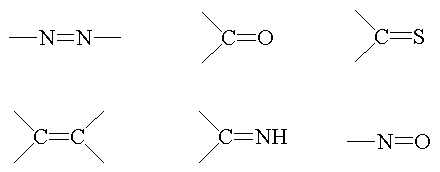
Some examples of chromophore chemical groups:

Some examples of auxochrome chemical groups:
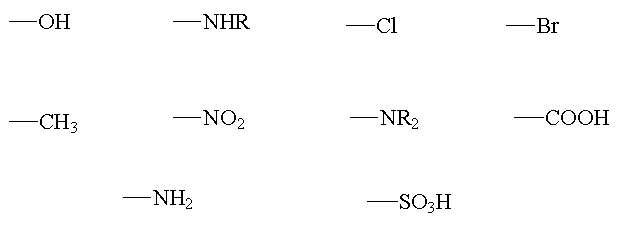
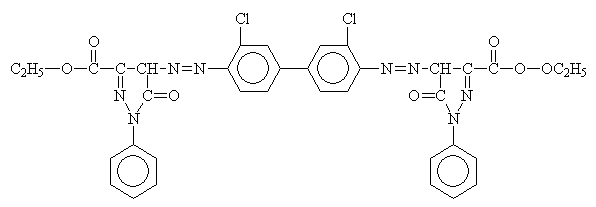
Organic pigments are divided into six categories:
  |
|
|
 |
|
|
Benzimidazolone Pigment:
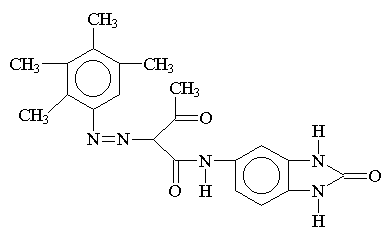
Hansa yellow
Phthalocyanine Pigments:
Phthalocyanine pigments are the single most successful class of organic pigments.
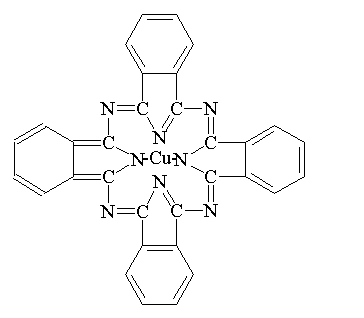
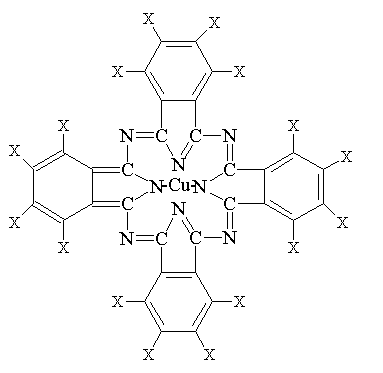
Phthalocyanine Green
(X = H, Cl or Br, empirical formula C32HxN8ClyBrzCu
x + y + z = 16)
Excellent stability to solvents, heat, light, and weathering
High tinctorial strength
Cost effectiveness
Consistency and uniqueness of shades
Completely non-toxic
![]() return to Pigments page
return to Pigments page