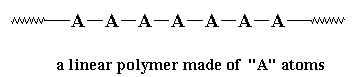

"Polymers Rule" for other reasons also. Polymers can have properties that small molecules never can. Take the synthetic polymer, ultra-high density polyethylene, for instance. Make it into a fiber, weave it into cloth and use it to make composite bullet resistant vests, and you save lives. Try to do that with a small molecule analog, candle wax. Yeah, wouldn't work the same, would it?
So that means there are fundamental differences between macromolecules and small molecules. Those differences go all the way from the molecular level to the nanoscopic and microscopic. In turn, these all impact the macroscopic level that we see and feel. We touched on some of these in the "Polymer Basics" page, but now it's time to dive in deeper.
So let's talk more about why polymers aren't as simple as small molecules starting at the molecular level. "It's complicated" just gets us started. It certainly is, but we have to look carefully at WHY it's complicated. What is it about polymers that are consequences of their huge size? And remember, we're talking mainly about how long the chains are, not how thick in cross sectional area. In truth, polymer molecules are about the same diameter as small molecules; it's just that they're so very, very long.
The first three rules are the same as from the introduction page. They're listed in the menu on the left, but we won't talk about them here much except when the lead to more intricate consequences, the "complicated" part.
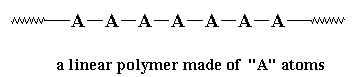
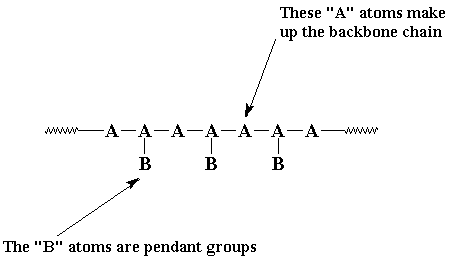
But usually we like to be more picky than that. Most of the time when we talk of polymers we're talking about molecules with molecular weights of hundreds of thousands, or even millions. We're also usually talking about linear polymers. A linear polymer is a polymer molecule in which the atoms are more or less arranged in a long chain. This chain is called the backbone. Normally, some of these atoms in the chain will have small chains of atoms attached to them. These small chains are called pendant groups. The chains of pendant groups are much smaller than the backbone chain. Pendant chains normally have just a few atoms, but the backbone chain usually has hundreds of thousands of atoms.
This unit of a carbon atom with two hydrogen atoms followed by a carbon atom with a hydrogen atom and a methyl group repeats itself over and over again along the backbone chain. This little recurring structure is called the repeat structure or the repeat unit.
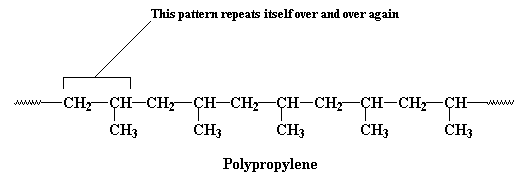
To make things simple, we usually only draw one unit of the repeat structure, like this:
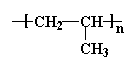
The repeat unit is put inside brackets, and the subscript n just stands for the number of repeat units in the polymer chain.
Polymers can come in other structures, though. To find out, take a look at the nonlinear polymer page.

For more on building polymers from monomers, go here.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |
