


Then you know what we do? We heat it again! This time we turn the heat up higher, and our carbon atoms kick off their hydrogens, and the rings become aromatic. This polymer is a series of fused pyridine rings.
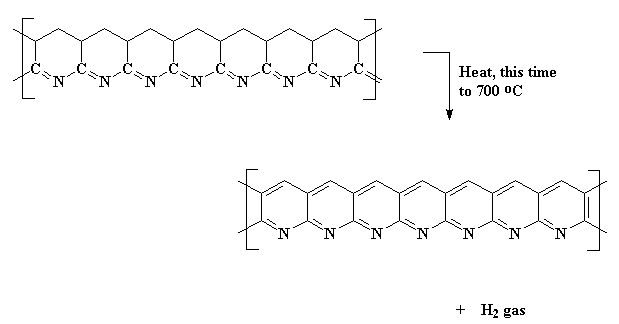
Then...guess what?...we heat it...AGAIN! Slow roasting the polymer some more at around 400-600 oC causes adjacent chains to join together like this:
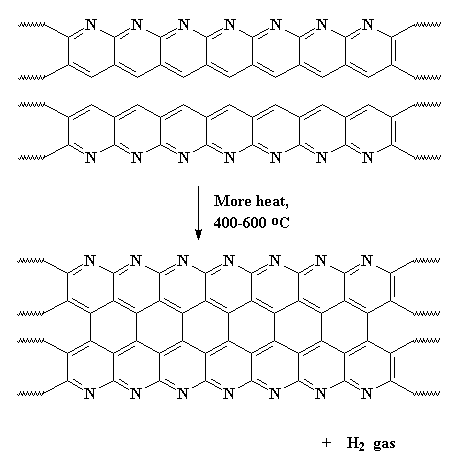
This expels hydrogen gas, and gives us a ribbon-like fused ring polymer. But don't think we're done yet! Next we crank up the heat, anywhere from 600 all the way up to 1300 oC. When this happens, we expel nitrogen gas and our newly formed ribbons will themselves join together to form even wider ribbons like those below. As you can see in the polymer we get, it has nitrogen atoms along its edges. These new wide ribbons can then merge to form even wider ribbons. As this happens, more and more nitrogen is expelled. When we're through, the ribbons are really wide, and most of the nitrogen is gone, leaving us with ribbons that are almost pure carbon in the graphite form. That's why we call these things carbon fibers.
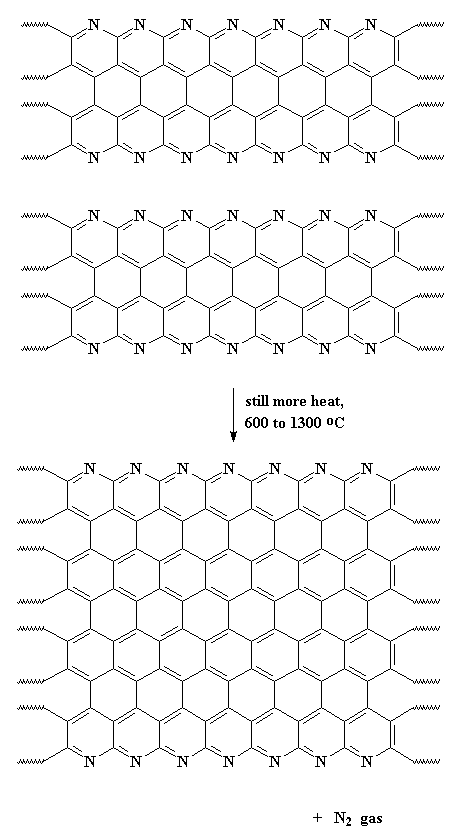
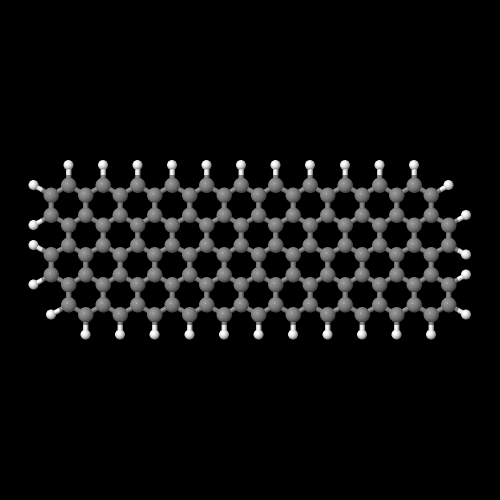
The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.

|
Return to Carbon Fiber |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
