This page is dedicated to rubber - the bouncy stretchy stuff that you find all over the place. Rubber is a name given to a lot of different polymers that are all elastomers. That means they can be stretched out and they will return to their original shape when you let go.
This page is dedicated to rubber - the bouncy stretchy stuff that you find all over the place. Rubber is a name given to a lot of different polymers that are all elastomers. That means they can be stretched out and they will return to their original shape when you let go.
The first rubber was the natural kind from the sap of hevea trees in Central America. We still use it today, but there are now several other kinds of rubber that people invented. They were "inspired" by natural rubber. Rubber actually got its name when people in Britain figured out that it could be used to erase or "rub out" mistakes made with a pencil. These little blobs of bouncy stretchy stuff used to rub out errors were called "rubbers." The British still call them that. So rubber was named after something that people made out of it.
To learn all kinds of neat stuff about rubber, go on our Rubber Expedition by clicking here. To learn about different kinds of rubber, read on!
One of the most well known natural polymers is polyisoprene, or natural rubber. Ancient Mayans and Aztecs, in what is now Central America, harvested it from the hevea tree and used it to make waterproof boots and the balls that they used to play a game similar to basketball.
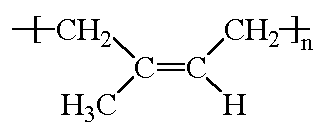
Polyisoprene is what we call an elastomer, that is, it recovers its shape after being stretched or deformed.
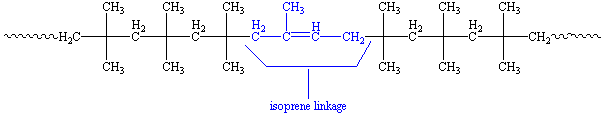
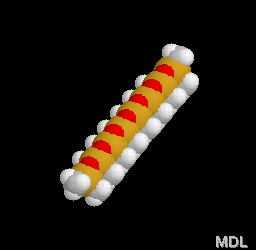
Try it! Take a rubber band - which is made of natural rubber - and stretch it. When you let go it'll be the same size as before. You can see in the 3-D model on the right how twisted and tangled the polyisoprene molecule is. When you stretch something made of rubber, the molecules all straighten out some, but when you let go they spring right bak into this twisted shape.
This is what the isoprene
These days natural rubber is treated to give it crosslinks, which makes it an even better elastomer. When rubber is crosslinked it won't melt when it gets hot. That's why rubber car tires don't melt when you drive really fast, even though they get very hot from friction with the road.
Another cool thing about polyisoprene is that people can make it almost as well as nature does. Our ability to imitate nature so closely is rare. Most natural polymers are much harder to copy.

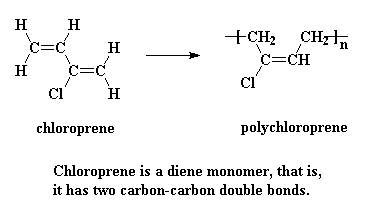
Polychloroprene is usually sold under the trade name Neoprene®. It's especially resistant to oil. It was the first synthetic elastomer, or rubber to be a hit commercially. It was invented by the Arnold Collins while working under the same fellow who invented nylon, Wallace Carothers, at DuPont.
Polychloroprene is made from the monomer chloroprene, believe it or not, like this:
Polybutadiene was one of the first types of synthetic elastomer, or rubber, to be invented. It's a lot like natural rubber, polyisoprene. Car parts like belts, hoses, and gaskets are made from polybutadiene because it stands up to cold temperatures better than other elastomers. Driving in the winter can be bad enough without hoses and gaskets going out on you!
Tire treads are often made of a hard rubber called poly(styrene-butadiene-styrene), or SBS rubber. It's a copolymer that contains polybutadiene.
Polystyrene is a tough hard plastic, and this gives SBS its durability. Polybutadiene is a rubbery material, and this gives SBS its rubber-like properties. Since SBS contains rubber and plastic, it acts like both materials.
It is an unusual material called a thermoplastic elastomer. These are materials which behave like rubbers at room temperature, retaining their shape after being stretched. But when heated they can be processed like plastics and molded into shapes. Most types of rubber are difficult to process because they are crosslinked. But SBS and other thermoplastic elastomers manage to be rubbery without being crosslinked, making them easy to make into stuff.
Polyisobutylene is a synthetic rubber, or elastomer. It is special because it is that only rubber that is gas impermeable, that is, it is the only rubber that can hold air for long periods of time. You may have noticed that balloons will go flat after a few days. This is because they are made of polyisoprene, and gases like air can gradually pass through it. Because polyisobutylene will hold air, it's used to make things like the inner liners of tires and the inner liners of basketballs.
Polyisobutylene was first developed during the early 1940s. Until then, everyone used polyisoprene. But in World War II Japan had taken over the world's rubber supply in Malaysia. So the United States and their allies developed polyisobutylene from a German process. This is interesting since we were also at war with Germany then. The scientists added just a little polyisoprene to the new rubber so that it could crosslink, like natural rubber does. This made it useful in the same ways natural rubber had been.
Because of the isoprene mixed in, polyisobutylene is a copolymer, and it looks like this:
About one or two out of every hundred repeat units is an isoprene unit, shown in blue. These have double bonds, which means the polymer can be crosslinked by vulcanization just like natural rubber. What is this vulcanization? To find out, click here.
Silicones are inorganic polymers because there are no carbon atoms in the backbone chain. (To chemists, "organic" means "it has carbon in it" and "inorganic" means it doesn't.)
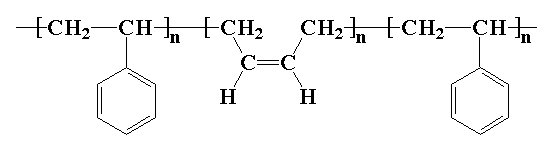
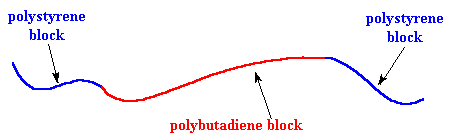
 Poly(styrene-butadiene-styrene), or SBS, is a hard elastomer that's used for things like the soles of shoes, tire treads, and other places where durability is important. It's a copolymer called a block copolymer made of three segments. The first is a long chain of polystyrene, the middle is a long chain of polybutadiene, and the last segment is another long chain of polystyrene. Here's a picture:
Poly(styrene-butadiene-styrene), or SBS, is a hard elastomer that's used for things like the soles of shoes, tire treads, and other places where durability is important. It's a copolymer called a block copolymer made of three segments. The first is a long chain of polystyrene, the middle is a long chain of polybutadiene, and the last segment is another long chain of polystyrene. Here's a picture:
Polyisobutylene
And this is that monomer isobutylene:
Silicone
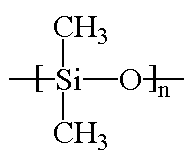 Silicones are used for a lot of things. They can be elastomers and lubricating oils. The caulking in your bathroom that seals the edges of the bathtub is probably made of a silicone. Silicones are also used to make the heat resistant tiles on the bottom of the space shuttle. Back on earth, silicones are used to make hair conditioners that don't cause buildup.
Silicones are used for a lot of things. They can be elastomers and lubricating oils. The caulking in your bathroom that seals the edges of the bathtub is probably made of a silicone. Silicones are also used to make the heat resistant tiles on the bottom of the space shuttle. Back on earth, silicones are used to make hair conditioners that don't cause buildup.
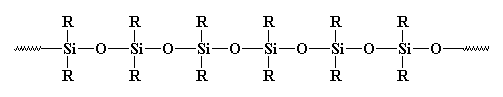 |
The backbone is a chain of alternating silicon and oxygen atoms. "R" stands for whatever molecules might be attached to the backbone. |
When different organic groups of atoms attach to the backbone, different kinds of silicones are formed. For example, if -CH3 (methyl) groups attach to the silicon atoms, the polymer is called polydimethylsiloxane. It's the most common silicone.
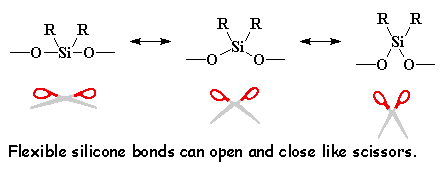
Silicones make good elastomers because the backbone chain is very flexible. The bonds between a silicon atom and the two oxygen atoms attached to it are very flexible. The angle formed by these bonds can open and close like a scissors without much trouble. This makes the whole backbone chain flexible.
Polydimethylsiloxane does something really strange when you mix it with boric acid, or B(OH)3. The mixture is soft and pliable, and you can mold it into any shape easily with your fingers. But it's also very bouncy. What's more, push it gently and it gives way, but hit it hard with a hammer and it cracks! Strangely, if you spread it over newspaper, and pull it away, it gets printed with a mirror image of the newspaper text. No industrial use was ever found for this wonder material, but tons of it have been sold as a toy called "Silly Putty."

|
Return to Kinds of Polymers |

|
Return to Main Page |