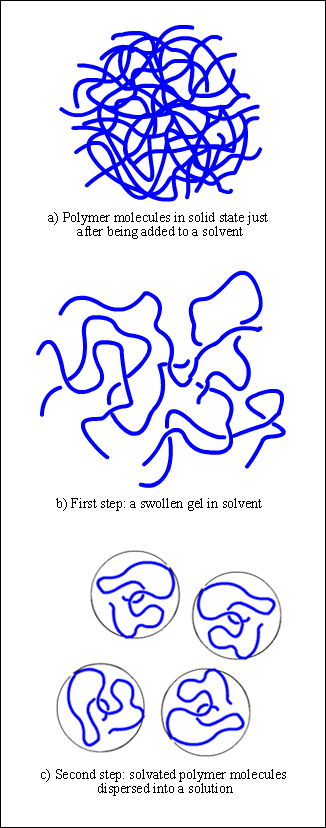
Figure 1. Schematic representation of the dissolution process for polymer molecules
Keywords
random coil,
hydrodynamic volume
As said earlier, the dissolution of a polymer is generally a slow process, which can take even several weeks, depending on the structure and the molecular weight of a given polymer.
When a low molecular weight solute such as sucrose is added to water, the dissolution process takes place immediately. The sugar molecules leave the crystal lattice progressively, disperse in water, and form a solution.
But polymer molecules are rather different. They constitute long chains with a large number of segments, forming tightly folded coils which are even entangled to each other. Numerous cohesive and attractive both intra and intermolecular forces hold these coils together, such a dispersion, dipole-dipole interaction, induction, and hydrogen bonding (Figure 1a).
Based on these features, one may expect noticeable differences in the dissolution behavior shown by polymers. Due to their size, coiled shape, and the attraction forces between them, polymer molecules become dissolved quite slowly than low molecular weight molecules. Billmeyer Jr. (1975) points out that there are two stages involved in this process: in the first place, the polymer swelling, and next the dissolution step itself.
When a polymer is added to a given solvent, attraction as well as dispersion forces begin acting between its segments, according to their polarity, chemical characteristics, and solubility parameter. If the polymer-solvent interactions are higher than the polymer-polymer attraction forces, the chain segment start to absorb solvent molecules, increasing the volume of the polymer matrix, and loosening out from their coiled shape (Figure 1b). We say the segments are now "solvated" instead of "aggregated", as they were in the solid state.

The whole "solvation-unfolding-swelling" process takes a long time, and given it is influenced only by the polymer-solvent interactions, stirring plays no role in this case. However, it is desirable to start with fine powdered material, in order to expose more of their area for polymer-solvent interactions.
When crystalline, hydrogen bonded or highly crosslinked substances are involved, where polymer-polymer interactions are strong enough, the process does stop at this first stage, giving a swollen gel as a result.
If on the contrary, the polymer-solvent interactions are still strongly enough, the "solvation-unfolding-swelling" process will continue until all segments are solvated. Thus, the whole loosen coil will diffuse out of the swollen polymer, dispersing into a solution. At this stage, the disintegration of the swollen mass can be favored by stirring, which increases the rate of dissolution.
However, once all the chain segments have been dispersed in the solvent phase, they still retain their coiled conformation, yet they are now unfolded, fully solvated, and with solvent molecules filling the empty space between the loosen segments. Hence, the polymer coil, along with solvent molecules held within, adopts a spheric or ellipsoid form, occupying a volume known as hydrodynamic volume of the polymer coil (Figure 1c).
The particular behavior shown by polymer molecules, explains the high viscosity of polymer solutions. Solvent and low molecular weight solutes have comparable molecular size, and the solute does not swell when dissolving. Since molecular mobility is not restricted, and therefore intermolecular friction does not increase drastically, the viscosity of the solvent and the solution are similar. But the molecular size of polymer solutes is much bigger than that of the solvent. In the dissolution process such molecules swell appreciably, restricting their mobility, and consequently the intermolecular friction increases. The solution in these cases, becomes highly viscous.
