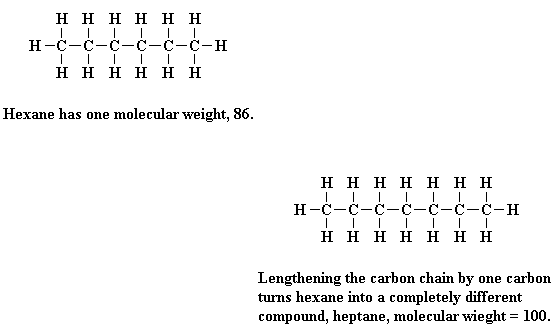

A whole page devoted to molecular weight? It might seem a bit strange to have a molecular weight page, but molecular weight is different for polymers than it is for small molecules. And we're talking about more than just the fact that polymers have really high molecular weights. Let us now explain:
Let's think about a small molecule, say, hexane. Hexane has a molecular weight of 86. Every hexane molecule has a molecular weight of 86. Now if we add another carbon to our chain, and the appropriate amount of hydrogen atoms, we've increased our molecular weight to 100.
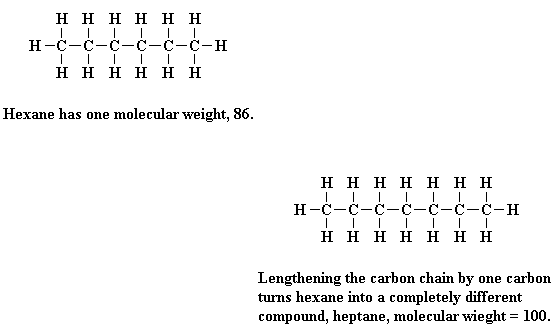
That's fine, but the molecule is no longer hexane. It's heptane! If we have a mixture of some molecules of hexane and some of heptane, the mixture won't act like pure heptane, nor will it act like pure hexane. The properties of the mixture, say, its boiling point, vapor pressure, etc., will be neither those of pure hexane nor pure heptane.
But polymers are different. Imagine polyethylene. If we have a sample of polyethylene, and some of the chains have fifty thousand carbon atoms in them, and others have fifty thousand and two carbon atoms in them, this little difference isn't going to amount to anything. If you really want to know the truth, one almost never finds a sample of a synthetic polymer in which all the chains have the same molecular weight. Instead, we usually have a bell curve, a distribution of molecular weights. Some of the polymer chains will be much larger than all the others, at the high end of the curve. Some will be much smaller, and at the low end of the curve. The largest number will usually be clumped around a central point, the highest point on the curve.
So we have to talk about average molecular weights when we talk about polymers. And we're not going to stop there. The average can be calculated in different ways, and each way has its own value. So let's talk about some of these averages, why don't we?
The number average molecular weight is not too difficult to understand. It is just the total weight of all the polymer molecules in a sample, divided by the total number of polymer molecules in a sample.
The weight average is a little more complicated. It's based on the fact that a bigger molecule contains more of the total mass of the polymer sample than the smaller molecules do.
A good way to understand the difference between the number average molecular weight and the weight average molecular weight is to compare some American cities.
Let's take four cities, say, Memphis, Tennessee; Montrose, Colorado; Effingham, Illinois; and Freeman, South Dakota. Now we'll take a look at their populations.

Now let's calculate the simple average population of the four cities:
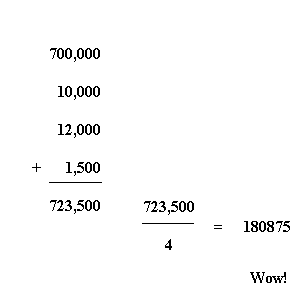
Now we see that of these four cities, that average population is 180,875.
But we could look at it a different way. Until now we've been worried about "the average city". What is the population of "the average city"? But let's forget about cities for a moment, and think about people. What size city does the average person among the populations of these four towns live in?
If you look at the numbers you can see that the average person doesn't live in a town of a population of 180,000. Take a look there. most of the people in the combined populations of the four towns live in Memphis, a town with a lot more than 180,000 people. So how do we calculate the size of town that the average person lives in, if the simple average doesn't work?
What we need is a weighted average. This is an average that would account for the fact that a large city like Memphis holds a larger percentage of the total population of the four cities than Montrose, Colorado. Doing this involves a little bit of math that looks scary but really isn't. All we do is take the total number of people in each city, then multiply that number by that city's fraction of the total population. Take all the answers we get for each city and add them up, and we get an answer that we'll call the weight average population of the four cities.
Let's walk through this to show what I mean. Take Memphis. It has a population of 700,000. The total population of our four cities is 723,500. So the fraction of people who live in Memphis is...
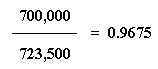
...0.9675, or we might say, 96.75% of the people live in Memphis. Now let's take our fraction, 0.9675, and multiply that by the population of Memphis:
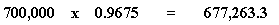
And we get an answer of 677,273.3. Now let's do the same thing for all the cities, and add up the answers:
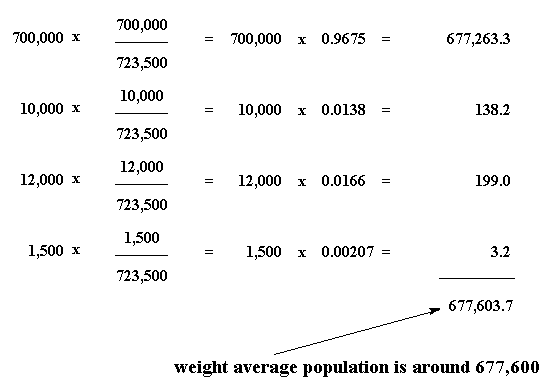
So our weight average population of the four cities is about 677,600. We can say from this figure that the average person lives in a city of about 677,600. That is more believable than saying that the average citizen lives in a city of 180,000.
We do the same thing with polymers. We calculate, with the same formula
as we used for the weight average population of our four cities, the
weight average molecular weight.
See, for example, the following activities:
Calculate the Molecular Weight of a Polymer here .
or
See an illustration of how molecular weight changes affect entanglement
using string
here .
Molecular weight can also be calculated from the viscosity of a polymer solution. The principle is a simple one: Bigger polymers molecules make a solution more viscous than small ones do. Of course, the molecular weight obtained by measuring the viscosity is a different from either the number average or the weight average molecular weight. But it's closer to the weight average than the number average. To read more about how we measure the viscosity average molecular weight, go read the dilute solution viscometry page.
With all these different molecular weights out there, things can get a little confusing. No single one of them tells the whole story. So it's usually best to try to know the molecular weight distribution. The distribution is a plot, like the one in the picture. It plots molecular weight on the x-axis, and plots the amount of polymer at a given molecular weight on the y-axis. Just for fun, we've shown you just where on the distribution curve the number, viscosity, and weight averages generally show up.
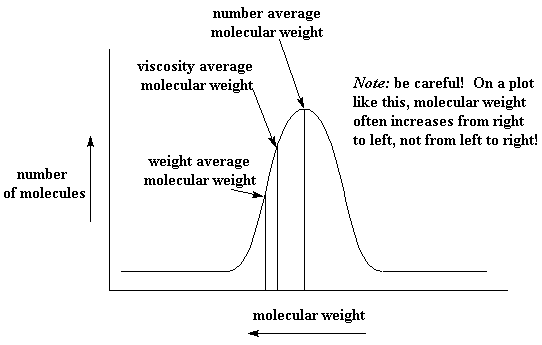
If we lived in a perfect world, where molecular distributions were always so nice and bell shaped, just knowing the averages might be enough. But they aren't always like that. Sometimes they are like this:
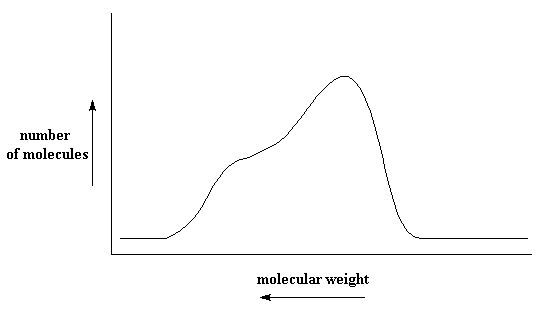
This kind of distribution can result from something called a Tromsdorff effect, which we find in free radical vinyl polymerization. Sometimes the distribution is even nastier, like this:
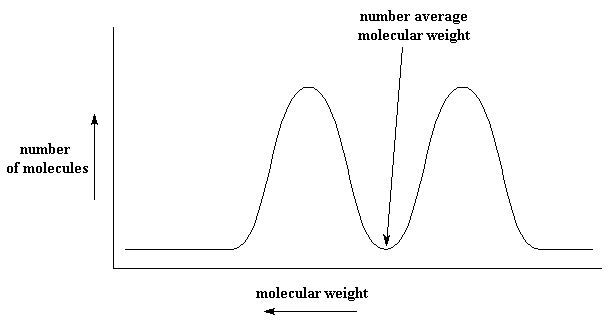
Here our number average molecular weight is a complete lie! There isn't a single molecule of that weight in the whole sample! Cases like these illustrate the need to know the complete distribution. The distribution can be given by a technique called size exclusion chromatography, and also by a new method called MALDI mass spectrometry.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |
