Parylene or PPX
aka Poly(p-xylylene)
Use your mouse on the model to rotate and zoom.
Click items below for other modifications.
spin on spin off
spacefill wire thick wire ball&stick
dots: Vanderwaals dots off
In your wildest dreams, did you ever envision making a high performance polymer by condensing a gas onto a surface? Be honest- probably not a process that would be high on your list of commercial polymer synthesis methods. But hold your horses, there IS such a polymer, and it's unique not just in how it's made, but in the unusual properties it has. So this month's "POTM" is unique for two reasons, making it a double first place winner (whatever that means in real life).
The polymer is made from the cyclophane monomer below which is sublimed and then thermally "cracked" to a very reactive neutral species called xylylene or 1,4-bismethylenecyclohexadiene. Generated in partial vacuum, the xylylenes condense on a surface (almost any surface) and spontaneously polymerize. The conformal thin film thus generated has very unusual properties- keep reading to find out all about it.
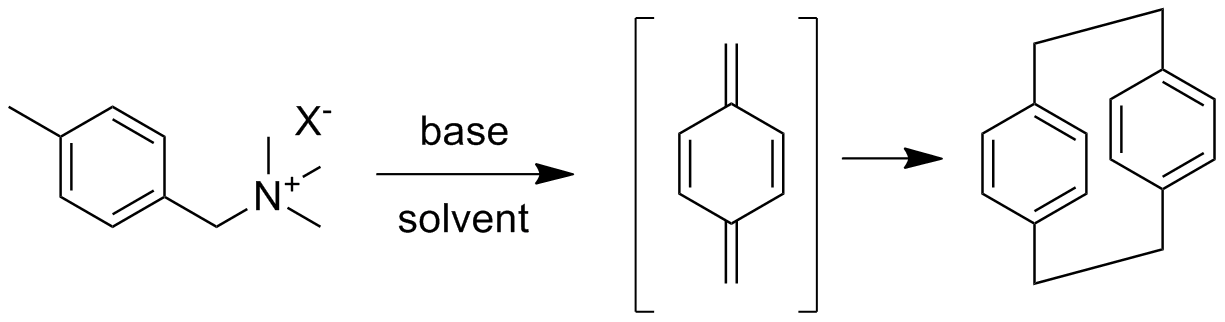
The overall polymer synthesis is shown below with the synthesis of the cyclic dimer "monomer" shown above. Both are unusual for a number of reasons. The cyclic dimer is made from a purified sample of p-xylene or p-dimethylbenzene. Halogenation followed by conversion to the trimethylammonium salt (one example) then allows Hoffman elimination with base in solution to give p-xylylene or 1,4-bismethylenecyclohexadiene. When two of these highly reactive species come together in a solvent, they react at one end to give a diradical (see below). Because the solution is dilute and there's nothing much else for the radicals to react with, they eventually react with each other or couple to give the cyclic dimer in good yield.

Now this dimer "monomer" is more stable than the p-xylylene, but still not totally happy- it has a lot of ring-strain caused by tying together the two aromatic rings face-to-face through the short -CH2CH2- groups. This forces the electron clouds happily circulating around each aromatic ring to be really close together and they don't like that. In fact, they repel each other. Just take a look at the molecular model below- looks strained, no? See the 3D model of this species by clicking on the image below and choosing "Vanderwaals" display. Given the chance then (say at 600 oC), the dimer breaks apart to give back the very reactive p-xylylene. To see a 3D model of p-xylylene, click here.

This xylylene intermediate has also been isolated and studied at very low temperature. But as you might expect, this species is very unhappy (i.e., reactive) and even at -78 oC undergoes spontaneous dimerization or even polymerization in solution. Why? Well, of course, to reform the much more stable aromatic ring. Remember: aromaticity is almost always good.
Back to the cyclic dimer, which is stable and easily stored. If you make the p-xylylene in the vapor phase, say at 600 oC, nothing much happens because of very low concentration. Any surface present that's at a lower temperature will be a site for condensation of xylylenes. And when two condensed molecules find each other, they immediately react. The intermediate diradical would really like to do anything rather than dimerize back to the strained "monomer," so it will now react almost instantaneously with other xylylene monomers to give polymer. This polymerization is fast and clean with no side-reactions or by-products. Neat!
Wait, there's more! You might be asking yourself, "So is this a step-growth or chain-growth polymerization process?" And the answer is (wait for it): YES! Not to be too cute (sorry, can't help it) but the mechanism has characteristics of both. First of all, it goes by way of radicals. Actually, they're diradicals, with one at each end of every propagating species. So any of the species present, from p-xylylene itself to the dimer, trimer and on up to the highest molecular polymer formed has two radical methylenes, one at each end. To re-emphasize a key point: even the monomer p-xylylene behaves as if it is a diradical.
Radical propagation also occurs in the most common and most used polymerization processes today- vinyl polymerization. The difference is this: in radical polymerization of a vinyl monomer (like styrene or methyl methacrylate), an active chain-end radical adds to a non-radical monomer (or the other way around if that helps you think about it). It does this in such a way that, while forming a new C-C bond, it also generates a new radical chain-end that looks and behaves just like the old one. Key point: two radical chain ends only react to terminate the polymerization, not to continue it.
Now with p-xylylene polymerization, reaction can occur between ANY two species present. And this is one characteristic of step-growth polymerization. In this case, however, each time radical coupling occurs to link up two of the methyenes to form an ethylene group between two benzene rings, it doesn't require monomer, just an end-group of anything else present. Oh, and here's the other key point: reaction of one end of any of the species present does NOT kill the radical at the other end. So basically, there is NO termination happening as long as there is sufficient chain-end mobility and concentration for two radicals to find each other. Theoretically, then, you could make infinite molecular weight polymer. Practical, you'll limited by basic thermodynamics involving chain-end mobility plus chain-end concentration. Both decrease as polymerization takes place until there's not enough of either for reaction to proceed. Things just stop, and any remaining radical chain-ends hang around until something comes along to kill them, like oxygen or an impurity or side-reaction of some kind.
 OK, now let's think about properties of these polymers. Most applications I've seen (in the
open literature as well as in patents) involve thin films of the polymer deposited on a surface.
In fact, as the picture to the right shows, even a natural rubber article (such as a baby bottle
nipple) can be coated with parylene. I got this particle example at a booth at a national
meeting of the American Chemical Society. You might be asking yourself, "So why would you
want to put a thin film of a pretty expensive polymer on the surface of such a mundane
polymer product?" Good question, and I have no idea. My guess is that it illustrated a
couple key properties of the parylene: first, it bonds well to surfaces like natural rubber AND
it is very flexible. You can squeeze and bend the nipple and the surface film doesn't break
or delaminate. I would guess that the polymerization process (remember those radical chain-ends?)
leads to chemical bonding, a C-C bond, between the growing parylene polymer and unsaturation (double
bonds) of the natural rubber. That means the film won't delaminate. Flexibility comes from the
film being so thin. Did you ever notice that even something as brittle as glass will bend
if you make it thin enough, like in fiberglass fibers and insulation? Ok, then, two key
properties of parylene films are adhesion and flexibility.
OK, now let's think about properties of these polymers. Most applications I've seen (in the
open literature as well as in patents) involve thin films of the polymer deposited on a surface.
In fact, as the picture to the right shows, even a natural rubber article (such as a baby bottle
nipple) can be coated with parylene. I got this particle example at a booth at a national
meeting of the American Chemical Society. You might be asking yourself, "So why would you
want to put a thin film of a pretty expensive polymer on the surface of such a mundane
polymer product?" Good question, and I have no idea. My guess is that it illustrated a
couple key properties of the parylene: first, it bonds well to surfaces like natural rubber AND
it is very flexible. You can squeeze and bend the nipple and the surface film doesn't break
or delaminate. I would guess that the polymerization process (remember those radical chain-ends?)
leads to chemical bonding, a C-C bond, between the growing parylene polymer and unsaturation (double
bonds) of the natural rubber. That means the film won't delaminate. Flexibility comes from the
film being so thin. Did you ever notice that even something as brittle as glass will bend
if you make it thin enough, like in fiberglass fibers and insulation? Ok, then, two key
properties of parylene films are adhesion and flexibility.
And here's another property that may have just zipped past you from the above discussion. If you were to ask (which you might if you're particularly alert right now), "So how long have you actually had that baby bottle nipple anyway?" I'd have to answer, "A long time, actually, amounting to more than a decade or two." Follow on question, of course, would be "Any changes in the parylene film?" And the answer would be, "Nope, not as far as I can tell." This dialog points out a key property, durability, that is important in lots of commercial applications. One that has been patented a few times and was recently touted in a trade magazine I read was using parylene films to protect electronic circuit boards. The trade journal compared treated and untreated circuit boards exposed to harsh salt water conditions. Guess which one showed almost no corrosion or other effects?
It also turns out that parylene films can be made amorphous or with controlled crystallinity. Let's think a little about why not being crystalline matters. Lots of polymers like nylons, PET polyester, polypropylene and good old polyethylene get their good thermal properties from crystalline domains. Those domains act like physical crosslinks, keeping the rest of the polymer, the amorphous part, from moving around, soft and gooey like (see discussion here on this). That amorphous, non-crystalline part is usually more than half to two-thirds of the total polymer so how it behaves is really important. It's even more important for polymers that don't have those crystalline crosslinks to hold everything in place. Key point: being able to control crystallinity means you can control various types of strength and toughness. So how do you actually do that? Another great question!

And just to remind you, while the melting transition involves long polymer chain segments freeing themselves from the crystalline lattice (at around the crystalline melting point, Tm), the amorphous phase undergoes a different type of thermal transition. It's called the glass transition, and it's always much lower then Tm. The molecules go from a glassy state (hence the name) to a rubbery or liquid-like state as it passes through Tg. The molecular motion associated is very different than for Tm, consisting of only short-range reorientations that involve just a few repeat units. Not only do those short-range motions make the polymer flexible, it usually makes it permeable to gases, maybe even to liquids to some degree.
So that leads us to another really interesting property of parylene films: gas and liquid impermeability. Yup, you heard (or read) that right. Parylene films have very low permeability to gases, and especially to liquids like water. And that's one of the main reasons that parylene films make such great protective coatings for materials sensitive to liquids and solution induced corrosion (such as from salt water). And just so you know, there have been a number of derivatives of the basic parylene structure made. These include the mono-chlorinated polymer (one Cl on every other aromatic ring), the tetrafluoro derivative (with fluorines replacing the aliphatic hydrogens on the methylene groups), plus a number of others. The only two commercial polymers I'm aware of are the unsubstituted parent and the chloro derivative."Where can I get some of this wonderful stuff?" you might ask. Great question, but you're missing a key point: you also need the apparatus used to actually lay down the parylene film on whatever you want to put it on. There are commercial suppliers who will sell you the cyclophane "monomer" but you're better off having them do the job for you, at least till you know you want to invest more in the process. Good luck and have fun!
References, to name just a few:
Errede, L.A.; Gregorian, R. S.; Hoyt, J. M.; "The Chemistry of Xylylenes. VI. The Polymerization of p-Xylylene," J. Am. Chem. Soc., 1960, 82 (19), pp 5218-5223; DOI: 10.1021/ja01504a048
Loeb, William E.; "Encapsulation by vacuum deposition of polymers," SPE Journal (1971), 27(9), 46-51
Joesten, B. L., "Thermogravimetry and differential scanning calorimetry of some poly-p-xylylenes containing halogen atoms," J. Applied Polym. Sci. (1974), 18(2), 439-48., DOI:10.1002/app.1974.070180211
Yeh, Y. S.; James, W. J.; Yasuda, H.; "Polymerization of p-xylylene derivatives. VI. Morphology of Parylene N and Parylene C films investigated by gas transport characteristics," J. Polym. Sci., Part B: Polym. Phys. (1990), 28(4), 545-68., DOI:10.1002/polb.1990.090280409