
One of the most common and useful reaction for making polymers is free radical polymerization. It is used to make polymers from vinyl monomers, that is, from small molecules containing carbon-carbon double bonds. Polymers made by free radical polymerization include polystyrene, poly(methyl methacrylate), poly(vinyl acetate) and branched polyethylene. But enough introduction. What is this reaction, and how does it work?

The whole process starts off with a molecule called an initiator.
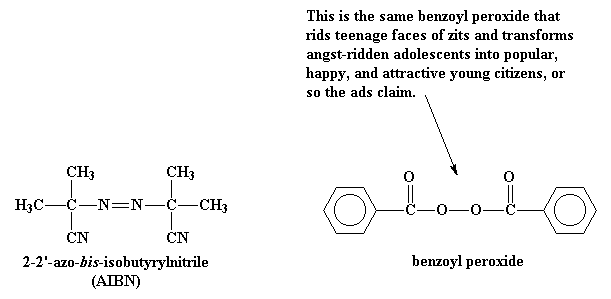
This is a molecule like benzoyl
peroxide or 2,2'-azo-bis-isobutyrylnitrile
(AIBN). What is special about these molecules
is that they have an uncanny ability to fall apart, in a rather
unusual way. When they split, the pair of electrons in the bond
which is broken, will separate. This is unusual as electrons
like to be in pairs whenever possible. When this split happens,
we're left with two fragments, called initiator fragments,
of the original molecule, each of which has one unpaired electron.
Molecules like this, with unpaired electrons are called free
radicals.
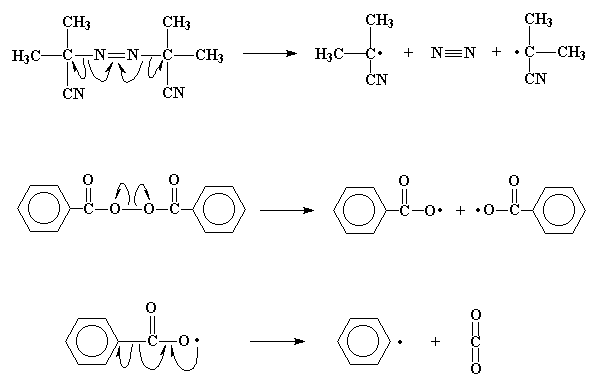
Now remember, these unpaired electrons will be quite discontent with
being alone and still want to be paired.
If they can find ANY electrons to pair up with, they will do so. The
carbon-carbon double bond in a vinyl monomer, like ethylene, has
a pair of electrons which is very easily attacked
by the free radical. The unpaired electron, when it comes near
the pair of electrons, can't help but swipe one of them to pair with
itself. This new pair of electrons forms a new chemical bond between
the initiator fragment and one of the double bond carbons of the
monomer molecule. This electron, having nowhere
else to go, associates itself with the carbon atom which is not
bonded to the initiator fragment. You can see that this will
lead us back where we started, as we now have a new free radical
when this unpaired electron comes to roost on that carbon atom.
This whole process, the breakdown of the initiator molecule to form
radicals, followed by the radical's reaction with a monomer molecule is
called the initiation step of the polymerization.
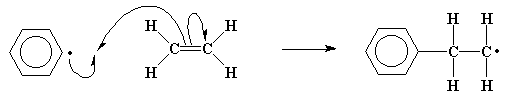
Wouldn't you know it, this new radical reacts with another ethylene molecule in the exact same way as the initiator fragment did. Of course, as we can see, this gets us nowhere as far as pairing electrons goes, because we always form another radical when this reaction takes place over and over again.
This process, the adding of more and more monomer molecules to the growing chains, is called propagation.
Because we keep remaking the radical,
we can keep adding more and more ethylene molecules, and build
a long chain of them.
Self-perpetuating reactions like
this one are called chain reactions.
So as long as the
chain keeps growing, who really cares if a few electrons remain
unpaired?

Sadly, the electrons care. Radicals are unstable, and eventually
they are going to find a way to become paired without generating
a new radical. Then our little chain reaction will come grinding
to a halt. This happens in several ways. The simplest way is
for two growing chain ends to find each other. The two unpaired
electrons then join to form a pair, and a new chemical bond joining
their respective chains. This is called coupling.
Coupling is one of two main types of termination reaction. Termination is the third and final step of a chain-growth polymerization. Initiation and propagation are the first two steps, of course.
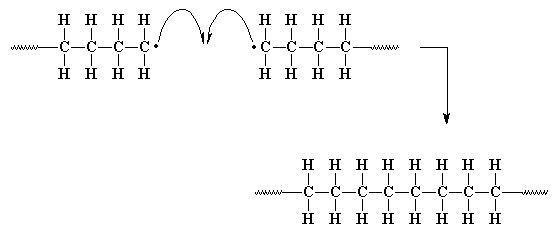
Now here comes the other termination reaction:
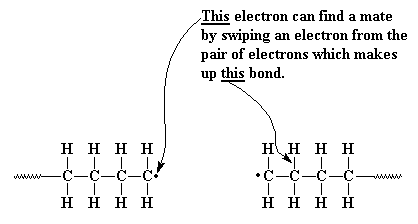
Another way in which our unpaired electrons can shut down
the polymerization is called disproportionation. This is a
rather complicated way in which two growing polymer chains solve the
problem of their unpaired electrons. In disproportionation, when two
growing chain ends come close together, the unpaired electron of one
chain does something strange. Rather than simply joining with the
unpaired electron of the other chain, it looks elsewhere for a mate. It
finds one in the carbon-hydrogen bond of the carbon atom next to the
other carbon radical. Our unpaired electron grabs not only
one of the
electrons from this bond, but the hydrogen atom as well. Now our first
chain has no unpaired electrons, the end carbon now shares eight
electrons, and everyone is happy.
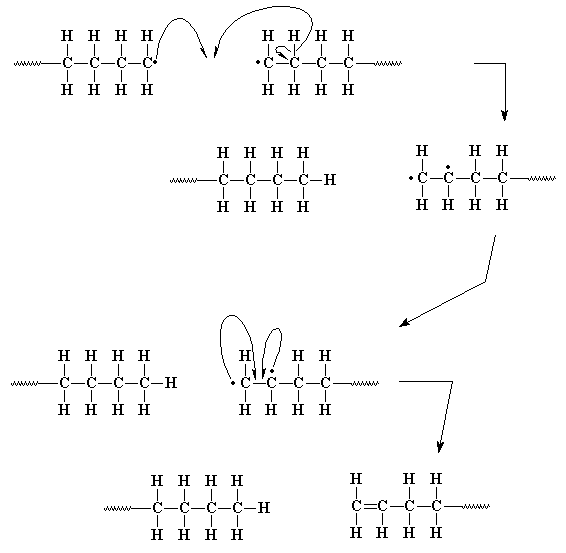
That is, except for the polymer chain which lost its hydrogen atom. It
now has not only one carbon atom with an unpaired electron, but two! Now
this looks bad but it's really not too difficult a problem, as it turns
out. The two
carbon radicals, being right next to each other, can easily join their
unpaired electrons to form a pair, and thus
chemical bond between the two carbon atoms. Now the two atoms already
shared one pair of electrons, and the second shared pair creates a
double bond at the end of the polymer chain.
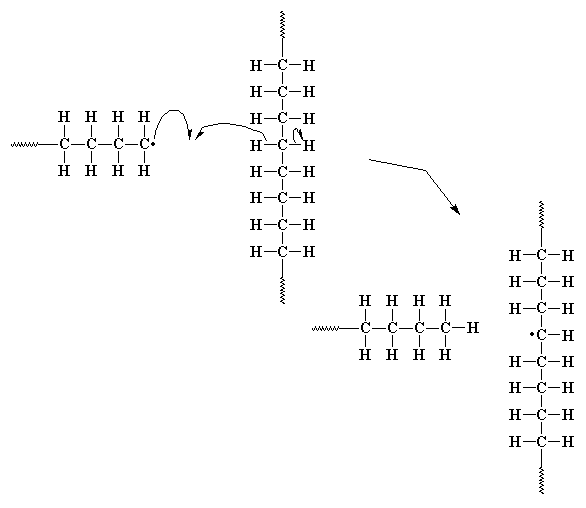
Sometimes, the unpaired electron at the end of a growing chain is so
unhappy that it will pair
itself with an electron from a carbon-hydrogen bond along the backbone
of another polymer chain. This leaves
an unpaired electron which is nowhere near the propagating chain end.
This electron can't form a double bond the way the electron from the last
example did, but it can and will react with a monomer molecule, just the
way the initiator fragment did. This starts a new chain growing out of
the middle of
first chain! This is called chain transfer to polymer, and the
result is a branched polymer. It is especially a
problem with polyethylene, so much that linear
non-branched polyethylene can't be made by free radical polymerization.
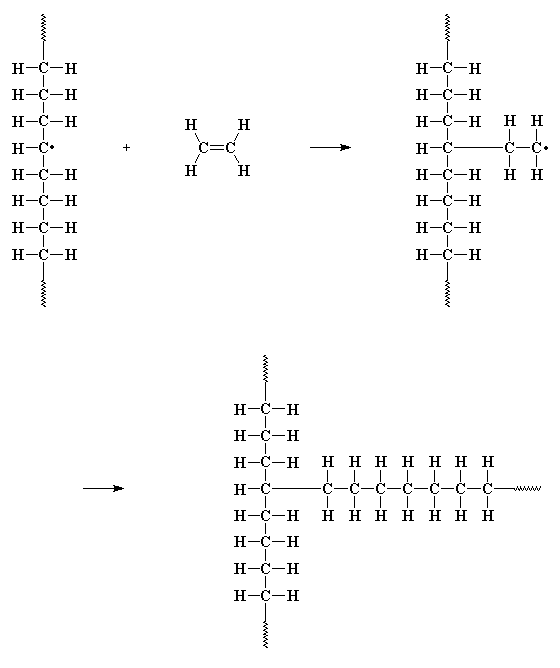
This branching has a big effect on how polyethylene behaves. To find out how, and how getting rid of branching helped make lowly polyethlyene better than Kevlar in bullet proof vests, visit the fun-filled, fact-filled polyethylene page.
 Return to Level Four Directory
Return to Level Four Directory Return to
Macrogalleria Directory
Return to
Macrogalleria Directory