Polyurethanes are the most well known polymers used to make foams. If you're sitting on a padded chair right now, the cushion is probably made of a polyurethane foam. But polyurethanes are more than just foam, lots more.
Polyurethanes are the single most versatile family of polymers there is. They are used for all kinds of things. Polyurethanes can be elastomers, and they can be paints or clear coat varnishes. One of the most useful coatings is something called "spar varnish," which gets its name from the exterior application it is used for- sail boat spars and masts. They can be fibers, and they can be adhesives such as "Gorilla Glue®," one of the few glues that bonds to just about anything (although it will foam if the surfaces aren't dry). They just pop up everywhere. A very unusual polyurethane is spandex which we'll talk about more later.
And here's one of the most important health related applications: thermoplastic elastomeric polyurethanes (also called TPU's) are turning up in all kinds of biomaterials. It seals in electronic devices, encases bioimplants such as pace makers and defibullators and has even been trialed in artificial hearts and heart valves. Who knows? One day soon, you might find a TPU device in you or one of your relatives.
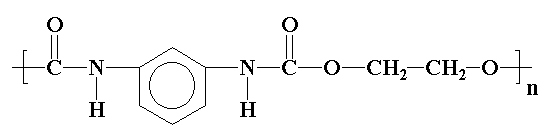
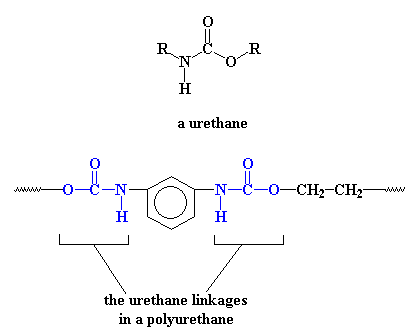
Now let's talk chemistry and polymer science, which is the real point of this site. It may not be obvious (or it just may) that polyurethanes are called polyurethanes because in their backbones they have a urethane linkage.
 |
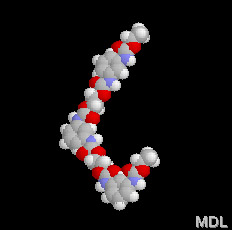
In the 3-D model you can see the urethane groups. Look for the red oxygen and blue nitrogen atoms. Each of those blobs is a urethane. |
The picture shows a simple polyurethane, but there are all kinds of ways to make polyurethanes, as long as they have a urethane linkage in the backbone. Some of them are very simple and some are more complex, like this one:
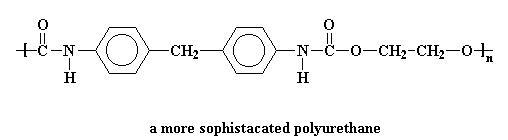
Polyurethanes can hydrogen bond very well, so they can be very crystalline. For this reason they are often used to make block copolymers with soft rubbery polymers. These block copolymers have properties of thermoplastic elastomers, which are polymers that can act like both a rubber and a thermoplastic. That means it can be thermally processed but then once it cools, it acts like an elastomer.
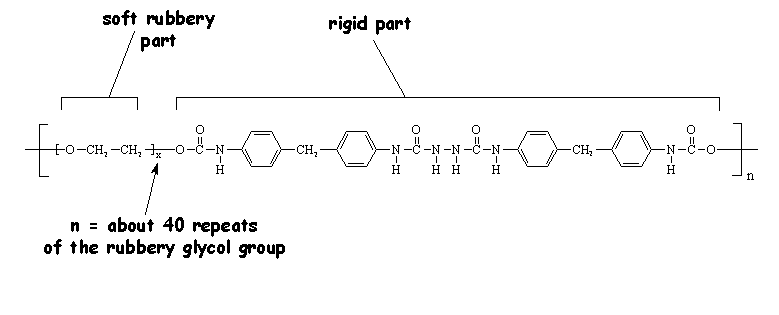
One unusual polyurethane thermoplastic elastomer is spandex, which DuPont sells under the trade name Lycra®. What gives spandex its special properties is the fact that it has hard and soft blocks in its repeat structure. The hard blocks, which contain the urethanes, bond tightly to the urethanes in other chains to form crosslinks of a sort. These crosslinks are thermally reversible, which means you can mold or extrude the material into fibers. When it starts to cool down, the physical crosslinks reform and give you back the elastomeric behavior.
And here's the really neat part. While the hard sections are all clumped together, the soft polyglycol sections of each chain stay rubbery, allowing the whole chian to stretch and flex. The result is a very strong and tough fiber or cloth that acts like an elastomer! So we use it to make exercise clothing like biker pants and swimsuits. It's also used in biomedical applications where the elastic properties coupled with biocompatibility make it important in medical devices.

|
Return to Kinds of Polymers |

|
Return to Main Page |
