
Basic Concepts
The image on the left is of the 3D model of nylon 6,6; on the right is nylon 6.
Just click on the image to view the model you can rotate and zoom in a new window.
Close it when you are ready to come back here.
For Nylon 6,6 at a glance, click here!
For Nylon 6 at a glance, click here!
Background
Commercial Importance
Nylons are some of the most important fibers produced commercially. If you've ever slept in a tent or used a toothbrush, you've used nylon fibers. But nylon can be more than just fibers. It's also used for self-lubricating gears and bearings. Nylon-clay composites are used to make under-hood automobile parts.
The two most important kinds of nylon are nylon 6,6 and nylon 6. These two nylons have almost identical properties. Both were invented in the late 1930s. Nylon 6,6 was discovered first. It was invented in the United States by Wallace Carothers who was working for DuPont.1 Not long after that Nylon 6 was invented in Germany by Paul Schlack who was working for I.G. Farben.2
Physical Properties
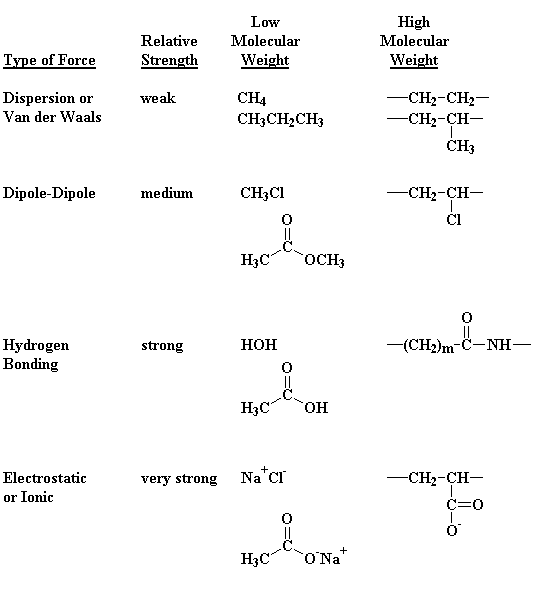
You may ask yourself, "Why does nylon act as it does?" You may ask yourself, "Why does nylon make such good fibers?" The answer to both is pretty simple: intermolecular forces. Just for review, Table 1 lists the different kinds of intermolecular forces. When we're talking about nylons, the most important intermolecular force is hydrogen bonding. The nitrogen-bonded hydrogens of one nylon chain will hydrogen bond very strongly with the carbonyl oxygens of another nylon chain. These hydrogen bonds make crystals of nylon very strong, because they hold the nylon chains together very tightly. Of course, these strong crystals make strong fibers.
Unless it has been drawn into fibers, only about 20-30% of the nylon in a given sample is crystalline when in solid form. The rest is in the amorphous phase. But even though it's non-crystalline, the chains are still bound strongly to each other by hydrogen bonds. This combination of crystalline and strongly associated amorphous phases is what makes nylon thermoplastics so tough. This only applies to nylons used as thermoplastics, mind you. When drawn into fibers, nylons become almost entirely crystalline.

We all know that a lot of the nylon produced ends up as clothing. But it also ends up as other everyday things like rope, tents, and toothbrush bristles. Sometimes nylon is used to make the belts that reinforce tires. Most passenger car tires have steel belts, but tires for aircraft, trucks and off-road vehicles are often made of nylon. Under the hood of your car you'll find nylon fibers reinforcing rubber belts, too.
Table 1
Nylon Nomenclature
There are several systems for naming nylons.3 There is the traditional system, the monomer system, and some newer systems, like the IUPAC method, which is the method Chemical Abstracts uses.4
In the traditional system, the name includes the word "nylon" followed by either one number or two numbers. If the nylon is made from an A-B monomer there will only be one number. But if there are two numbers, then you know that the nylon was made from an A-A/B-B monomer system. For nylons made from A-B monomers, the number tells you how many carbon atoms are in the monomer, which of course is also the number of carbon atoms in the repeat unit of the nylon. Hence, if a nylon is named "nylon 6", you know that it is made from an A-B monomer, and that A-B monomer has six carbon atoms. For nylons made from A-A/B-B monomer systems, the two numbers tell you how many carbon atoms are in the diamine monomer, and how many carbons are in the diacid or diacid chloride monomer. For example, if your nylon is called "nylon 6,10", you know that it is made from an A-A/B-B monomers system, you know that the diamine from which it was made has six carbons, and that the diacid or diacid chloride from which it was made has ten carbon atoms.
The monomer system, also called source based nomenclature, is not used very often. It is confusing because different monomers can be used to make the same nylon. For example, you can polymerize e-caprolactam to get poly(e-caprolactam), and you can polymerize aminohexanoic acid to get poly(aminohexanoic acid). But in fact, you get the same polymer each time: nylon 6. So don't waste a lot of time worrying about this system. just know how to interpret its names so you won't be confused if you ever come across them.
Finally, there are the structure-based names. The two main ones are the IUPAC and the non-IUPAC systems. Both attempt to unambiguously describe the repeat unit, but the non-IUPAC is much simpler.
Take a look at Table 2 to see examples of all of these nomenclature systems.
Table 2
Naming Conventions for Alkyl Polyamides5

Naming Conventions for Alkyl Polyamides5

Theory
Step-growth Polymerizations and Chain-growth Polymerizations
All polymerizations fall into two categories: step-growth polymerizations and chain-growth polymerizations. Both step-growth polymerizations and chain-growth polymerizations are used to make nylons. Making nylon from a diacid and a diamine is a step-growth polymerization. So is making nylon from an amino acid. Making nylon from lactams is usually a chain-growth polymerization.
So what is the difference between the two types of polymerization? That would take a long time to explain, so if you want to know, go read the Macrogalleria page called Putting Them Together. But there are some practical differences you should know about for this experiment...
In a step-growth system, we start off with monomers. The monomers combine and grow into dimers, trimers, tetramers, and so forth. The molecules get bigger and bigger, but only when we're done (when the polymerization reaches high conversion) do we have high molecular weight polymers.
But in a chain growth system, we start off with monomers, and the monomers quickly form high molecular weight polymers. There are high molecular weight polymers present in your test tube just after you start the polymerization. What's more, you won't have dimers, trimers, and other oligomers hanging around. A growing polymer chain grows so fast that it reaches high molecular weight quickly, and it doesn't spend any real length of time as an oligomer.
Polycondensations
Please don't get confused, but there is another way to describe polymerizations other than the step-growth/chain-growth system. There is also the condensation/addition system. To know more about this system, again go visit the Macrogalleria page Putting Them Together. The most important thing you need to know about this system is that it classifies all polymerizations as polycondensations or polyadditions. Polycondensations are polymerizations in which a small molecule by-product is produced. The by-product is usually something like water, HCl, or once in awhile NaCl. Polyadditions on the other hand are polymerizations in which no by-product is produced.
We're going to talk now about using polycondensations to make nylons. The simplest polycondensation for making nylons is the polymerization of a diacid and a diamine. This reaction might not normally go to high conversions, but by removing the water by-product (usually by carrying out the reaction under vacuum so the water evaporates), we can force this reaction go to higher conversions.
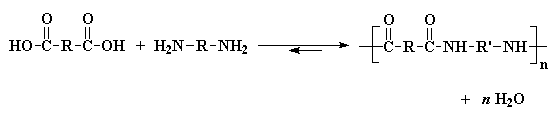
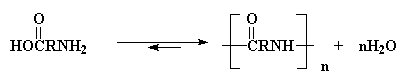
AB systems have an advantage over AA-BB systems. The advantage is that in an AB system, one always has the same amount amine groups and acid groups. As we all know, stoichiometric balance of amine and acid groups is absolutely critical when making nylons. With AA-BB systems, the amounts of the two monomers must be measured very carefully to ensure perfect stoichiometric balance.
Interfacial Polymerizations
Making nylon 6,6 is even easier if you use a diamine and a diacid chloride instead of a diacid. This is because acid chlorides are much more reactive than acids. The reaction is done in a two-phase system. The amine is dissolved in water, and the diacid chloride in an organic solvent. The two solutions are placed in the same beaker. Of course, the two solutions are immiscible, so there will be two phases in the beaker. At the interface of the two phases, the diacid chloride and diamine can meet each other, and will polymerize there. There is special way to do this called the "Nylon Rope Trick"6, 7, and we'll show you how to do that on this page.
While this is a neat party trick, it isn't used commercially because, first, acid chlorides are a lot more expensive than acids, and second, acid chlorides stink horribly, and are much more toxic than acids. And third, the fibers produced by this trick aren't very strong, anyway.
Ring-Opening Polymerization
The ring opening-polymerization of lactams is a chain-growth polymerization. It is also a polyaddition reaction, that is, no byproducts are produced. The thermodynamic driving force for ring-opening polymerizations is ring strain. Cyclic molecules polymerize in order to relieve the strain. Take a look at Table 3, and you'll see that 5- and 6-membered rings don't have very much ring strain, so they don't polymerize well. But 7-membered rings, like e-caprolactam, are much more strained and polymerize easily. As you can see in Table 3, so do many larger cyclic monomers.
Table 3
Polymerizability of Lactams8
| Type of | Order of Polymerizability |
| Polymerization | (ring sizes) |
| anionic (strong base) | 7 > 5 > 6 |
| hydrolytic (water initiated) | 7 > 8 > 9 >> 5 > 6 |
| cationic | 8 > 7 > 11 > 5 > 6 |
There are two ways to carry out a ring-opening polymerization of e-caprolactam. Down at the nylon factory, nylon 6 is made using a water-initiated process. Read about it on the Macrogalleria page Making Nylon 6.
The second way to make nylon 6 is to use a strong base as an initiator.9 To find out more about the anionic polymerization of caprolactam, go to this page.
References
1. Carrothers, W.H. (to E.I. DuPont de Nemours and Co.), U.S. Patent 2,130,523 (1938).
2. Schlack, P., German Patent 748,253 (1938), U.S. Patent 2,241,321 (1941). 3.a. B. F. Greek, Chem. Eng. News, May 30, 1983, p.14; b. Chem. Eng. News, April 18, 1983, p. 6.
4. R. B. Fox, J. Chem. Educ., 1974, 51, 41 and 113.
5. B. Odian, Principles of Polymerization, 2nd edition, John Wiley & Sons, Publishers, New York, 1981, p. 12.
6. P. W. Morgan and S. L. Kwolek, J. Chem. Educ., 1959, 36, 1982.
7. B. Z. Shakhashiri, Chemical Demonstrations, Vol. 1, University of Wisconsin Press, Madison, Wisconsin, p. 213.
8. H. R. Allcock and F. W. Lampe, Contemporary Polymer Chemistry, Prentice-Hall, Inc. Englewood Cliffs, N.J., 1981, pp. 128-129.
9. Procedures and References cited in L. J. Mathias, J. Chem. Educ., 1983, 60, 990.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |