 |
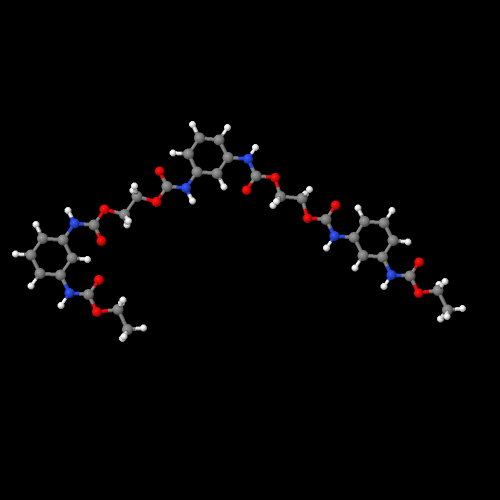
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Polyurethanes are the most well known polymers used to make foams. If you're sitting on a padded chair right now, the cushion is more than likely made of a polyurethane foam. Polyurethanes are more than foam.
Much more than foam!
Polyurethanes are the single most versatile family of polymers there is. Polyurethanes can be elastomers, and they can be paints. They can be fibers, and they can be adhesives. They just pop up everywhere. A wonderfully bizarre polyurethane is spandex.
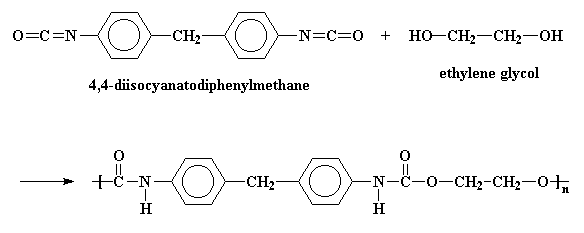
Of course, polyurethanes are called polyurethanes because in their backbones they have a urethane linkage.
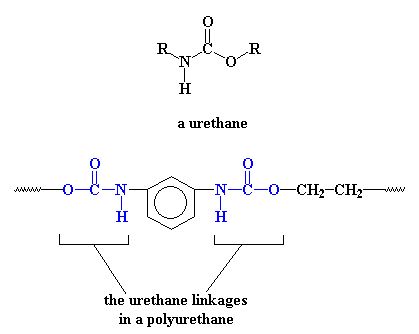
The picture shows the a simple polyurethane, but a polyurethane can be any polymer containing the urethane linkage in its backbone chain. More sophisticated polyurethanes are possible, for example:
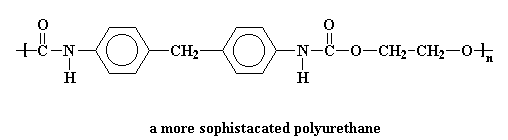
Polyurethanes are made by reacting diisocyanates with di-alcohols. To find out how, click here.
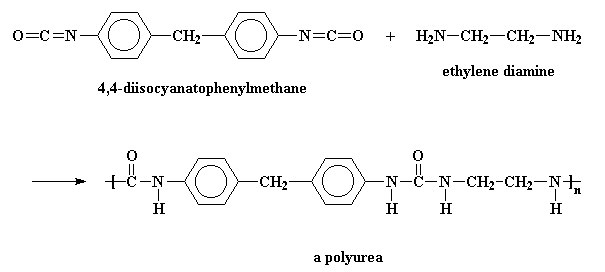
Sometimes, the dialcohol is replaced with a diamine, and the polymer we get is a polyurea, because it contains a urea linkage, rather than a urethane linkage. But these are usually called polyurethanes, because they probably wouldn't sell well with a name like polyurea.
Polyurethanes can hydrogen bond very well, and thus can be very crystalline. For this reason they are often used to make block copolymers with soft rubbery polymers. These block copolymers have properties of thermoplastic elastomers.
Spandex
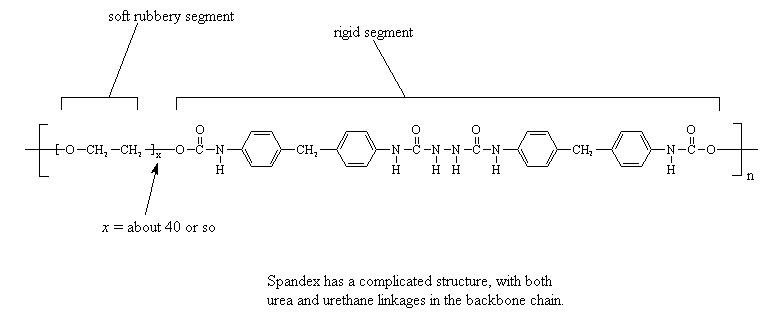
One unusual polyurethane thermoplastic elastomer is spandex, which DuPont sells under the trade name Lycra. It has both urea and urethane linkages in its backbone. What gives spandex its special properties is the fact that it has hard and soft blocks in its repeat structure. The short polymeric chain of a polyglycol, usually about forty or so repeats units long, is soft and rubbery. The rest of the repeat unit, you know, the stretch with the urethane linkages, the urea linkages, and the aromatic groups, is extremely rigid. This section is stiff enough that the rigid sections from different chains clump together and align to form fibers. Of course, they are unusual fibers, as the fibrous domains formed by the stiff blocks are linked together by the rubbery soft sections. The result is a fiber that acts like an elastomer! This allows us to make fabric that stretches for exercise clothing and the like.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |