
What Crosslinking Is
All About...and Why
Keywords
elastomer,
gel,
thermoset
Stealing Vulcan's Fire
There was a time long past when the only rubber we had was natural rubber latex, polyisoprene. Straight out of the tree, natural rubber latex isn't good for much. It gets runny and sticky when it gets warm, and it gets hard and brittle when it's cold. Tires made out of it wouldn't be much good unless one lived in some happy land where the temperature was seventy degrees year round.A long time ago...how long, you ask? It was about a hundred and sixty years ago, 1839 to be exact. This was before there were any cars to need tires, but the idea of a useable rubber was still attractive. One person trying to make rubber more useful was named Charles Goodyear, a tinkerer and inventor, and by no means a successful one at this point. While goofing around in his kitchen with a piece of fabric coated with a mixture of rubber latex, sulfur and a little white lead, he accidentally laid it on a hot stove top. It began sizzling like a mass of really smelly bacon or (strangely enough) burning rubber. Wouldn't you know, when he took a look at this mass of rubber, he found it wouldn't melt and get sticky when it was heated, nor would it get brittle when he left it outside overnight in the cold Massachusetts winter. He called his new rubber vulcanized rubber. His research into rubber crosslinking took years and he borrowed more money than he could ever pay back. This left him destitute despite the tremendous importance of his discovery.
The Goodyear Tire & Rubber Company's
famous blimp above Philadelphia.

Goodyear's name would be mud with creditors, but it was still marketing gold. More than one
company will adopt Goodyear's name, two of which will survive into the twenty-first century. The first
is the small west coast firm Goodyear Rubber and Supply, which would purchase the name
 from the Goodyear estate soon after Goodyear's death. The second is the much more famous
company founded by Frank A. Seiberling
in East Akron, Ohio in 1898. Seiberling will name his company in honor of Goodyear, and it is
still known as The Goodyear Tire & Rubber Company today. Speaking of tires, the tire business
will be ready to explode in 1898, because of a new contraption called an automobile.
from the Goodyear estate soon after Goodyear's death. The second is the much more famous
company founded by Frank A. Seiberling
in East Akron, Ohio in 1898. Seiberling will name his company in honor of Goodyear, and it is
still known as The Goodyear Tire & Rubber Company today. Speaking of tires, the tire business
will be ready to explode in 1898, because of a new contraption called an automobile.
Tying it All Together
What had happened here? What did the sulfur do to the rubber? What it did was it formed bridges. Which tied all the polymer chains in the rubber together. These are called crosslinks. You can see this in the picture below. Bridges made by short chains of sulfur atoms tie one chain of polyisoprene to another, until all the chains are joined into one giant supermolecule.
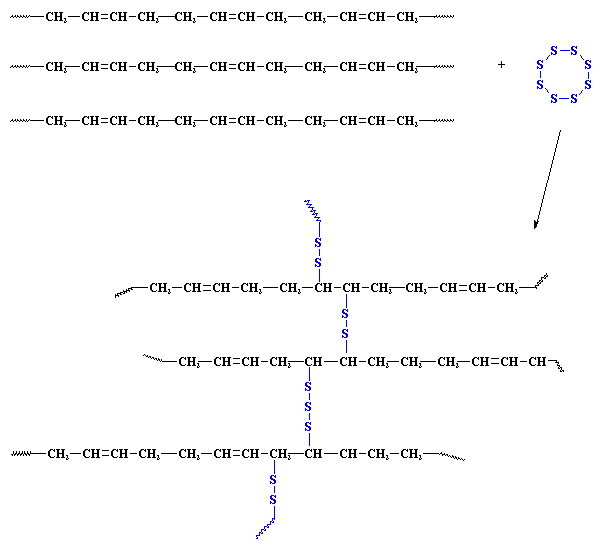
Yes, folks, this means exactly what you think it does. An object made of a crosslinked rubber is in fact one single molecule. A molecule big enough to pick up in your hand.
Wow!
These crosslinks tie all the polymer molecules together. Because they are tied together, when the rubber gets hot, they can't flow past each other, nor around each other. This is why it doesn't melt. Also, because all the polymer molecules are tied together, they aren't easily broken apart from each other. This is why the Charles Goodyear's vulcanized rubber doesn't get brittle in when it gets cold.
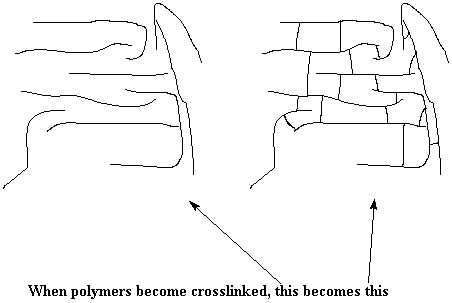
We can look at what's going on conceptually, and take a look at the bigger picture. The drawing below shows the difference between a lot of single uncrosslinked polymer chains, and a crosslinked network.
Other kinds of rubber, which chemists call elastomers that can be crosslinked include:
More! More!
But rubber isn't the only thing that can be crosslinked. Plastics are also made stronger by crosslinking. Formica is a crosslinked material and the material used in many plastic "glass" for screen doors (for example) or in bathtubs surrounds are crosslinked acrylates and methacrylates. One common crosslinker is EGDMA (shown below). Even contact lenses are crosslinked using special (and expensive) difunctional monomers like the one shown below as well.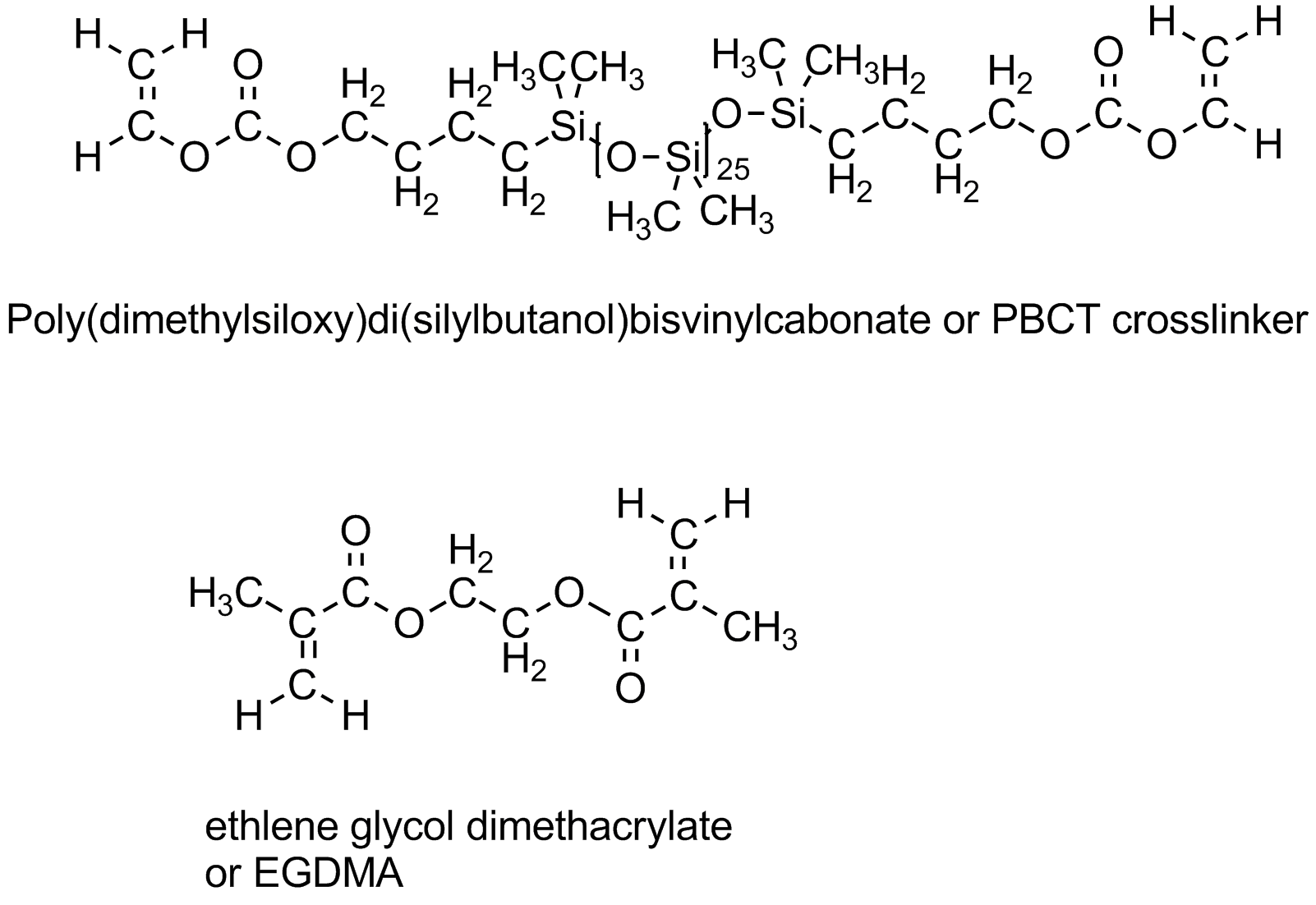
This crosslinker also contains silicone units that help transport oxygen from the air into the eye through the lens. Very important to keep you eye healthy if you wear contacts. For more information on contact lenses, go to the Polymer of the Month entry here.
Crosslinked polymers are usually molded and shaped before they are crosslinked. Once crosslinking has taken place, usually at high temperature, the object can no longer be shaped. Because heat usually causes the crosslinking which makes the shape permanent, we call these materials thermosets. This name distinguishes them from thermoplastics, which aren't crosslinked and can be reshaped once molded. Interestingly, the first thermoset was again polyisoprene. The more sulfur crosslinks you put into the polyisoprene, the stiffer it gets. Lightly crosslinked, it's a flexible rubber. Heavily crosslinked, it's a hard thermoset. (It was Charles' brother Noah who made the first polyisoprene thermosets, by the way.) Here are some crosslinked thermosets:
Crosslinked polymers can also be coatings, adhesives, and electronic parts. Crosslinked materials can't dissolve in solvents, because all the polymer chains are covalently tied together. But they can absorb solvents. A piece of a crosslinked material that has absorbed a lot of solvent is called a gel. One kind of gel you may be familiar with is crosslinked polyacrylamide. Uncrosslinked polyacrylamide is soluble in water, and crosslinked polyacrylamides can absorb water. Water-logged gels of crosslinked polyacrylamides are used to make soft contact lenses.
The Catch
Crosslinking makes both elastomers and plastics stronger, but there is a problem. Because crosslinked materials don't melt, it is very hard to recycle them. One answer to this problem is to create crosslinks that can be reversed, or undone, believe it or not. One family of materials using reversible crosslinks are thermoplastic elastomers
Epilogue
Charles Goodyear never got rich from his invention. He spent his life in lawsuits over patent infringement. But after he died, a company founded in his name, Goodyear, became the successful corporate giant we know today. The company is now centered in Akron, Ohio. I say this because across town, at another rubber company, B.F. Goodrich, someone else came up with a clever new way to crosslink rubber. Click here to find out what it was.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |