via Anionic Chain-growth Polymerization
Keywordsanion
ring-opening polymerization
What's in a Name??
Nylon 6 is an awful lot like our friend nylon 6,6. You can look at the picture if you don't believe me.
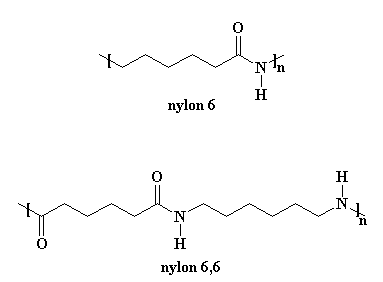
But making nylon 6 is lot different from nylon 6,6. First of all, nylon 6 is only made from one kind of monomer, a monomer called caprolactam. It's a strained cyclic ring with a pre-formed amide group that will become the amide in the repeat unit of the polymer. Take a look at the structure below- at least you now know what the name "caprolactam" stands for.

Nylon 6 can be made in two ways, one used industrially and the other used mostly for research purposes although it has potenetial... The commercial method involves heating caprolactam to about 250 oC with about 5-10% water thrown in. So what happens to caprolactam when there's water around? There are a number of steps involved, all of which you can see in detail by going to this page. But be sure to come back here to get...
"The Rest of the Story"
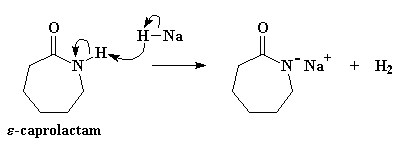
Enough small talk. Let's get on with the business of turning caprolactam into nylon 6 using an anion of some kind. The propagating anion is actually formed from caprolactam itself by reaction with a strong base. How strong a base? Very strong. A normal strong base like NaOH isn't going to work here. We'll need an extra strong base like sodium hydride (NaH). The hydride anion is an incredibly strong base and doesn't form any reactive by-product that can interfere with our polymerization. The key is that when it sees caprolactam, it runs straight to the amide hydrogen and pulls it right off, as you can see in Figure 1.
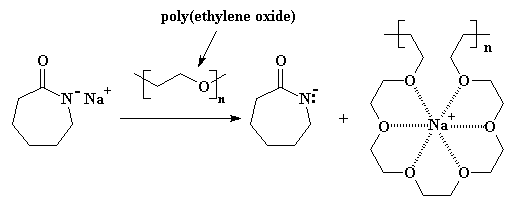
Fortunately for this reaction (and lots of others, actually), a new type of complexing agent was discovered a few years back: crown ethers. These cyclic multi-ether molecules are great at complexing with cations like sodium. Only problem is, they're not easy to make, which means they're pretty expensive. What to do? What to do?
Turns out some smart scientists (or maybe just lucky ones) realized that the main chain (or backbone) of polyoxyethylene [also called poly(ethylene oxide)] looks very much like a crown ether. That is, if you tilt your head a little and close one eye... This POE will complex the sodium cation, keeping it from associating with the anionic nitrogen, and making the reaction go cleaner and faster. You can see this in Figure 2.
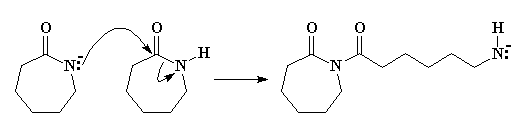
So the nitrogen, free to react, will donate an unshared pair of electrons to the carbonyl carbon of another caprolactam molecule. (Remember, carbonyl carbons are electron deficient, and are easily attacked by anions.) After some electron-shuffling in which the electrons in the bond between the carbonyl carbon and the amide nitrogen shift to the nitrogen, the second caprolactam molecule ring-opens, as you can see in Figure 3.
The new molecule formed also has a negatively-charged nitrogen, an amine anion. This is an unstable species, so the activation energy is high for this step. This makes this the slow step of the reaction. For this reason, there is an induction period at the beginning of the reaction before polymerization begins.
Being unstable, that anionic nitrogen will abstract a proton from another molecule of e-caprolactam, as Figure 4 clearly shows.
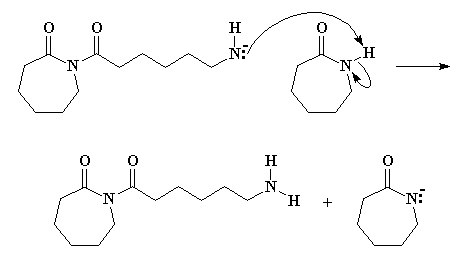
We have an anion of e-caprolactam once again, and the negatively charged nitrogen attacks the ring carbonyl carbon of the species we just formed. Take a look at Figure 5 and you'll see what's happening.
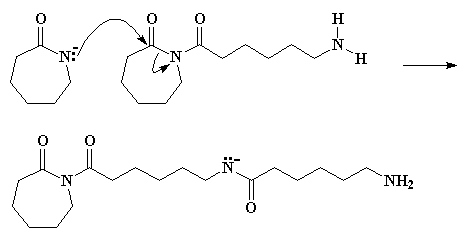
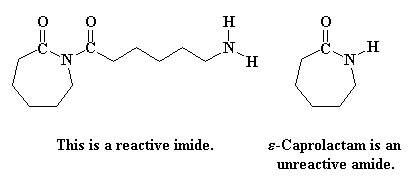
This step is faster than the that first ring opening step, the one in Figure 3, because the starting species involved this time is much more reactive. You see, the reacting compound this time is an imide, a compound with a nitrogen atom bonded to two carbonyl carbons. The last time we were reacting an amide, which is a compound with a nitrogen bonded to only one carbonyl carbon. Imides are much more reactive than amides.
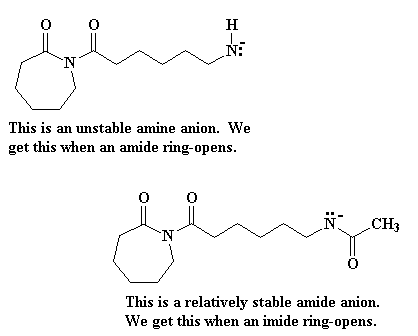
Part of the reason why imides are more reactive than amides is the fact that when an imide ring-opens we get an amide anion, whereas the product of an amide ring-opening is an amine anion. Amine anions are very unstable, but amide anions are more stable because the negative charge is delocalized on to the carbonyl group bonded to the nitrogen atom.
Of course, this gives us another unstable nitrogen negative charge, and it takes a proton from another e-caprolactam molecule, which then adds to the growing chain in the same way as we saw before. This keeps happening over and over until we get high molecular weight nylon 6.
Just one more thing...remember that the first ring-opening step was so slow? We can get around this slow step by throwing a little bit of N-acetylcaprolactam into the reaction mixture. Not only is this a reactive imide, just like the rings in our growing chain, but it produces an amide anion when it ring-opens rather than an unstable amine anion. It takes the place of e-caprolactam in the first step, so the slow step is eliminated, and so is that annoying induction period.
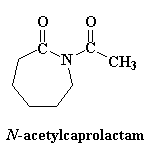
So the upshot of the insightful observation on POE (which is how we talk about polyoxyethylene among polymer scientists) plus the high initiation reactivity of N-acetylcaprolactam means that if we add them along with the caprolactam and heat, it makes the polymerization faster and gives high molecular weight nylon 6.
And here's the sum effect: after heating the mixture for 2-3 minutes, Bingo! High molecular polymer you can reach in (not with your fingers though), grab a little and pull out a fiber than can be stretched sometimes as long as dozens of meters (that's yards for all you die-hard non-metrics). Just be careful not to heat the polymer mixture longer than it takes (which you figure out by doing it a few times) because your polymer will thermally degrade, the molecular weight will drop, and you won't be able to draw good fibers.
Tested Nylon 6 Synthesis
Now if you'd actually like to make nylon 6 the way it's (not) made industrially, we have a procedure for you to follow. It uses caprolactam similar to the method discussed above. You use anionic chain-growth polymerization to make nylon 6 in a test tube really fast and to really high molecular weight. It's simple, quick and efficient. Only drawback is that you need to do it in a lab, where an instructor can buy the NaH initiator you need, and to make sure you're careful and doing it right. Oh, well, nothing in life is without some risk and road bumps...Click here to see the procedure and here to download a copy.
Nylon 6 NMR Spectra
Ok, so you're wildly successful at the procedure above and now have a sample of what you're pretty sure is nylon 6. How do you confirm that hypothesis? Easy! Take an NMR spectrum or two. But wait! You need a standard to compare it to, right? Have we got a deal for you: both proton and carbon spectrum of commercial nylon 6.Go here to view a 1H spectrum and and here to see the 13C spectrum.

|
Return to Nylon Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
