Welcome to the world of
High Performance Polymers!


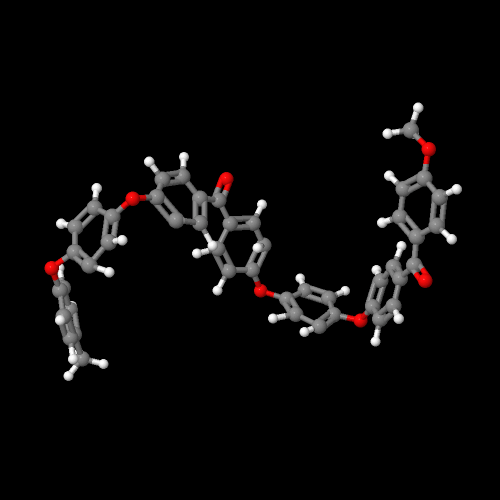
The model on the left above is PEK, on the right of PEEK
You can just click on either image to pop up a 3D version.
Be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
High performance polymers are called that because, you guessed it, they have exceptional physical and thermal properties. The two main families of the ones described here have aromatic groups (benzene rings) linked by a combination of one or two ether oxygens alternating with a ketone group.
Oddly enough (or not since we scientists are pretty dull when it comes to naming things), these two polymers are called poly(ether ketone) or PEK and poly(ether ether ketone) or PEEK. The names could fool you since they make no mention of the benzene rings that have to be there. The figure above left is for PEK (one ether, one ketone with two intervening benzene rings) and the one on the right is for PEEK, the polymer with two ethers and one ketone (plus three intervening benzene rings). Click on either image and you can view a 3D version that you can rotate and zoom.
One of the most interesting things about these polymers is how they're made.
Their syntheses involve a type of reaction called "nucleophilic aromatic substitution"
or NAS for short. Don't we scientists just love acronyms?
Oddly enough, the mechanism involves nucleophile attack on a substituted carbon of an
aromatic ring. That kind of attack is relatively easy on a primary or secondary carbon
of a hydrocarbon chain or at an allylic or benzylic carbon with a good leaving group.
What's surprising is that it can happen on an aromatic ring. And in fact, it's actually a different mechanism completely even though we use the same name for both.
"So how is it different exactly?" Great question! We're getting into some fairly
advanced organic chemistry, but that's good. Let's talk about the details.
First, it's different in that it's really a two-step process, also called an "addition-elimination" mechanism. Normally the SN2 (acronym for substitution-nucleophilic-second-order) reaction involves a one-step process with a single transition state. That is, the attacking nucleophile approaches the substituted aliphatic carbon and smoothly replaces the leaving group, all in one smooth process with only one transition state.
In NAS, on the other hand, the attacking group adds to the substituted aromatic carbon (as in the first step below) to give a special kind of resonance-stabilized intermediate sometimes called a "Meisenheimer Complex." That addition step has it's own transition state that leads to the complex.

Now one of two things can happen to that complex. It can pop off the attacking group and go back to what it was before, which actually gets you nowhere in terms of what you want. Or the substituent that was already there can pop off, leaving the new attacking group in its place. This step also goes through its own transition state. So for this NAS mechanism, there are two transition states and one intermediate that exists for a finite lifetime. That intermediate can actually be observed in some cases. With SN2, there's only a single transition state and no intermediate that forms, so there's no way to actually "see" that process.
There are two requirements for NAS to occur. First, you need a good leaving group, which oddly enough does NOT follow the same order as for SN2 reactions. Halides are the normal leaving group in both NAS and SN2. For the alkyl and allyl halides, the order of leaving group ability is I > Br > Cl (with F being pretty much a non-starter).
Here's a strange thing: for NAS, the order is F > Cl with Br coming in a distant third and I not working at all. So what's the deal here? The explanation is apparent once you look at the intermediate for NAS. Here, the F (and Cl to some extent) is very electronegative. That makes the approach of the nucleophile (the methoxide anion shown here) easier because it finds the fluorine carbon attractive. Why? Because the fluorine likes extra electron density around itself and pulls it from the carbon it's attached to, kind of like movies stars attracting paparazzi.
Great, but that's only half the story. The other half is the stabilizing group in the ortho and/or para position to the halide leaving group. If you look at the intermediate above, the negative charge that gets pushed into the benzene ring resonates around but likes to stay next to the very electronegative carbon next to the nitro group. In fact, there are two more resonance structures with the negative charge actually at one or the other of the two oxygens of the nitro group and a double bond between the nitrogen and the ipso (attached) carbon. And you should know by now that the more resonance structures you can draw for such an intermediate, the more stable it is.
So now we're ready for "the rest of the story" on NAS. In short, the monomers used to make PEK, PEEK and PEKK (Ah! Surprised you with that one, didn't I? Can you guess what it's structure is?) all have either F or Cl leaving groups AND the ketones function as stabilizing groups for the NAS intermediate. In fact, you can even draw a resonance structure with a double bond from the ketone carbon into the aromatic ring. That puts the negative charge out onto the carbonyl oxygen which just loves all the attention (remember that movie star?). Now does this weird addition-elimination process make sense? Hope so, because it's also the basis for other reactions involving substitution on aromatic rings. Stay tuned for the rest of that story!

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
