hydrodynamic volume
This page is about how we measure the molecular weight of polymers. Now before you read this page, it's best that you read the molecular weight page first. If you haven't read it, you can go there by clicking here. Then come back and read this wonderful page.Now, has everyone read the molecular weight page? You have and you loved it and thought it was the most fabulous page you've ever read? Great!
Remember that when we're dealing with a polymer, we're not talking about a single molecular weight, but a distribution of molecular weights, like you see in the picture below. The best we can do is to talk about an average molecular weight, and to describe the distribution of molecular weights around the average. In English that means counting up how many polymer molecules in a sample really have the average molecular weight, and how many are higher and lower, and by how much.
That's exactly the information that this picture tells us. It gives us the number average molecular weight Mn at the top of the curve, and how many polymer molecules are actually at that molecular weight, and how many polymer molecules are at different molecular weights. (See that arrow on the x-axis pointing to the left? That's not a mistake - the higher molecular weights are on the LEFT on this graph. Keep on reading and you'll see why...) 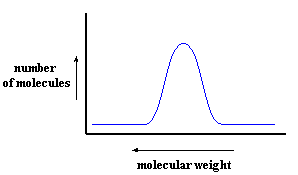
That's a downright useful graph, sure enough, but how does one get a graph like this? We get them using SEC. Now you Wall Street high rollers will know that SEC can stand for the federal Securities and Exchange Commission, and you college football fans will know SEC stands for the South Eastern Conference, but SEC here doesn't stand for either. Here it stands for size exclusion chromatography.
So how does this fabulous technique work? That will take some explaining, but it goes something like this: First you dissolve the polymer, usually in a solvent called tetrahydrofuran, or THF for short. Click on the molecule if you want to see what it looks like in 3-D! Then we take this solution of the polymer and we shoot it through a tube. We call this tube a column even though it isn't straight and vertical, but wound into a spiral coil. This isn't just any tube. It's a tube filled with little tiny beads.
But these aren't just any beads. The beads are made of crosslinked polystyrene. It's crosslinked to keep it from dissolving in the THF. Also the beads are filled with little tiny holes or pores. The pores come in all different sizes. Some are great big holes, and some are itty-bitty ones.
This is important to know, because SEC would not work if the holes were all the same size. The holes have to be different sizes. This is why: when you shoot the polymer solution into the column, the polymer molecules get distracted on their way through. The chains get caught up in the holes in the beads. They won't stay there forever, mind you. A polymer molecule will get caught up in one hole, then come out, pass on down the tube a little way, then get caught in another pore. It'll stay there a moment, then come out and travel a little further till it finds another pore it likes...you get the idea. Eventually it makes it to the end of the column.
Eventually. But eventually doesn't happen at the same time for each polymer molecule. What I'm trying to say here is that some polymer molecules take longer than others to get through the column. Big polymer molecules with higher molecular weights can't fit in some of the smaller holes. Because there are fewer pores that the big ones can get caught in, these molecules pass through the column pretty quickly. But smaller polymer molecules with lower molecular weights can fit into the small pores. Because they can fit in more pores, they get caught in more pores, obviously. So they take longer to get through the column.
It may help to think of an adult and a child walking through toy store. The adult will walk through fairly quickly, but the child is going to get distracted by all the cool toys, stopping to look and play with each one. It's going to take the kid a lot longer to walk through the toy store than it will the adult. Polymers are the same way. The bigger ones pass through the column uninterested in the pores in the beads, but the little ones in their curiosity have to stop to check out every little pore. So the big molecules pass through the column a lot faster than the smaller ones.
Putting a number on it
As a matter of fact, when a size exclusion chromatograph (that's what we call the machine we use to perform SEC) is calibrated correctly, we can know the molecular weight of a polymer just based on the time it takes to pass, or elute through to column. What's more, we have detectors that can count how many polymer molecules are coming out of the end of the column at a given time. So, we can make a plot of time on the x-axis and the number of polymer molecules coming out at a given time on the y-axis, like this:Because we can calculate molecular weight from elution time, we can turn this plot into a plot of molecular weight on the x-axis and the number of molecules with a particular weight on the y-axis, like this: 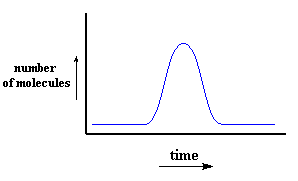
Now remember, the higher the molecular weight, the shorter the time it takes for the polymer molecule to pass through the column. So on this plot, molecular weight decreases from left to right. That's just the opposite of what you'd expect, so be careful and keep this in mind when you're looking at SEC plots. 
And just in case you're wondering what these wonderful size exclusion chromatographs look like, I've thrown in a picture of Kevin, a young polymer chemist extrordanaire, working on one. The column is in the little black box behind his head. The gray box at the far left is the pump that shoots the polymer solution through the column.

Drawbacks
There is a drawback to SEC. In SEC, we're really not measuring mass so much as the hydrodynamic volume of the polymer molecules, that is, how much space a particular polymer molecule takes up when it's in solution. We can approximate the molecular weight from SEC data because we know the exact relationship between molecular weight and hydrodynamic volume for polystyrene, and we use polystyrene as a standard. But the relationship between hydrodynamic volume and molecular weight isn't the same for all polymers, so we get only an approximate measurement.But don't give up hope. There is a new method that can measure molecular weight averages and molecular weight distributions very exactly. Go read about it. It's called matrix-assisted laser desorption/ionization mass spectrometry.


