IntroductionFor extra background information, visit these fun-filled Macrogalleria pages:
Polyelectrolyte Expansion - General Evidence
Experimental
Measurement of Intrinsic Viscosity
Appendix
Polyelectrolytes
Dilute Solution Viscosity
Polyelectrolyte Expansion - General Evidence
Both light scattering and viscosity studies provide direct evidence for expansion of polyelectrolytes in solution. In Figure IX-1, the polymer radius of gyration of polymethacrylic acid is plotted as a function of the degree of dissociation of the carboxyl groups (a).Note that in general (`Rg2 )1/2 increases as a increases.
Now, we can also use light scattering to measure increases in (`r2 )1/2 or (`Rg2 )1/2 as salt is added to an aqueous polyelectrolyte solution as is shown in Figure IX-2. Obviously as counterions are added (Na+) which can diffuse into the polymer matrix or domain, they greatly effect the repulsion between negatively charged carboxylic acid groups. The result is to decrease (`Rg2 )1/2 as [Na+ Cl-] increases.
The radius of gyration or (`Rg2)1/2 is readily calculated from the slope of each plot.
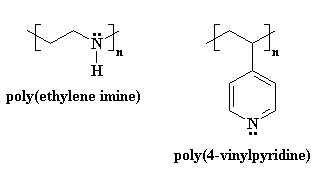
In considering viscometric analysis of aqueous solutions of polyelectrolytes we can consider extrapolation of hsp /C vs. C to very dilute solutions where the counterions diffuse into the bulk of the solution at more dilute concentrations. This leaves the fixed charges on the polyion with lower shielding and the polyion expands. Thus, we see the dramatic results in Figure IX-4. Now we can add (or rather consider) one other complication in viscosity measurements due to shear dependence:
Region I - [h] varies as salt concentration changesRegion II - reflects decreasing interaction between chains as you get more dilute.
Region III - as yet more dilute counterions diffuse into solution and polyion expands thus hsp increases C.
Experimental
This experiment will be conducted with poly(acrylic acid).1. Prepare at least four dilutions of poly(diallyldimethylammonium chloride) in 100 ml of deionized water.2. Neutralize the dissolved poly(diallyldimethylammonium chloride) to a pH of 7 with 3M NaOH solution.
3. Fill a viscometer with an aliquot of the solution. Leave enough volume to allow for dilution in the viscometer.
4. Measure the apparent viscosity as a function of concentration while diluting in the viscometer. Plot the reduced specific viscosity (hsp/c) as a function of concentration. Continue to measure viscosities until the plot of reduced specific viscosity against concentration goes through a maximum upon dilution.
5. Determine the slope of the line in region 1. For a rigid rod the Mark-Houwink constant should theoretically be 2. For a free-draining coil, the Mark-Houwink constant should be 1.
6. Conduct the same set of viscosity measurements as above, but now in 1M NaCl. Draw a plot of reduced specific viscosity against concentration and extrapolate to zero concentration to obtain the intrinsic viscosity.
Question
How would you check if the molecules were fully extended rigid rods, using viscosity data alone?Measurement Of Intrinsic Viscosity
For polyelectrolytes, the intrinsic viscosity is measured in an excess of salt solution. The salt shields the polyions from intramolecular expansion-- and a linear plot should be obtained.
Find the appropriate Mark-Houwink constants from the Polymer
Handbook and calculate the molecular weight of your sample of poly(acrylic
acid).
As the solution is diluted, chemical potential forces counterions to
diffuse away from the polygon. Thus at low polymer concentrations many
unshielded charged groups exist on the polyion chain, and the polyion
expands due to intrachain charge repulsion.
Appendix

 Return to
USM Polymer Science Online Laboratory Directory
Return to
USM Polymer Science Online Laboratory Directory